Bottom Line Up Front: Stanford’s CRISPR-GPT achieves 99% planning accuracy and enables complete novices to perform successful gene editing in hours, not months. This AI breakthrough signals America’s dominance in biotech automation while China races to catch up.
The future of medicine arrived on July 30, 2025, when Stanford University published groundbreaking research in Nature Biomedical Engineering. Furthermore, CRISPR-GPT represents more than just another AI tool—it’s a complete paradigm shift that democratizes genetic engineering, much like how AI is revolutionizing drug discovery across the pharmaceutical industry.
Imagine transforming an undergraduate student with zero genetics experience into a successful gene editor in just one day. Moreover, this isn’t science fiction—it’s documented reality, backed by rigorous peer review from four of the world’s most prestigious institutions.
The Shocking Numbers Behind CRISPR-GPT’s Success
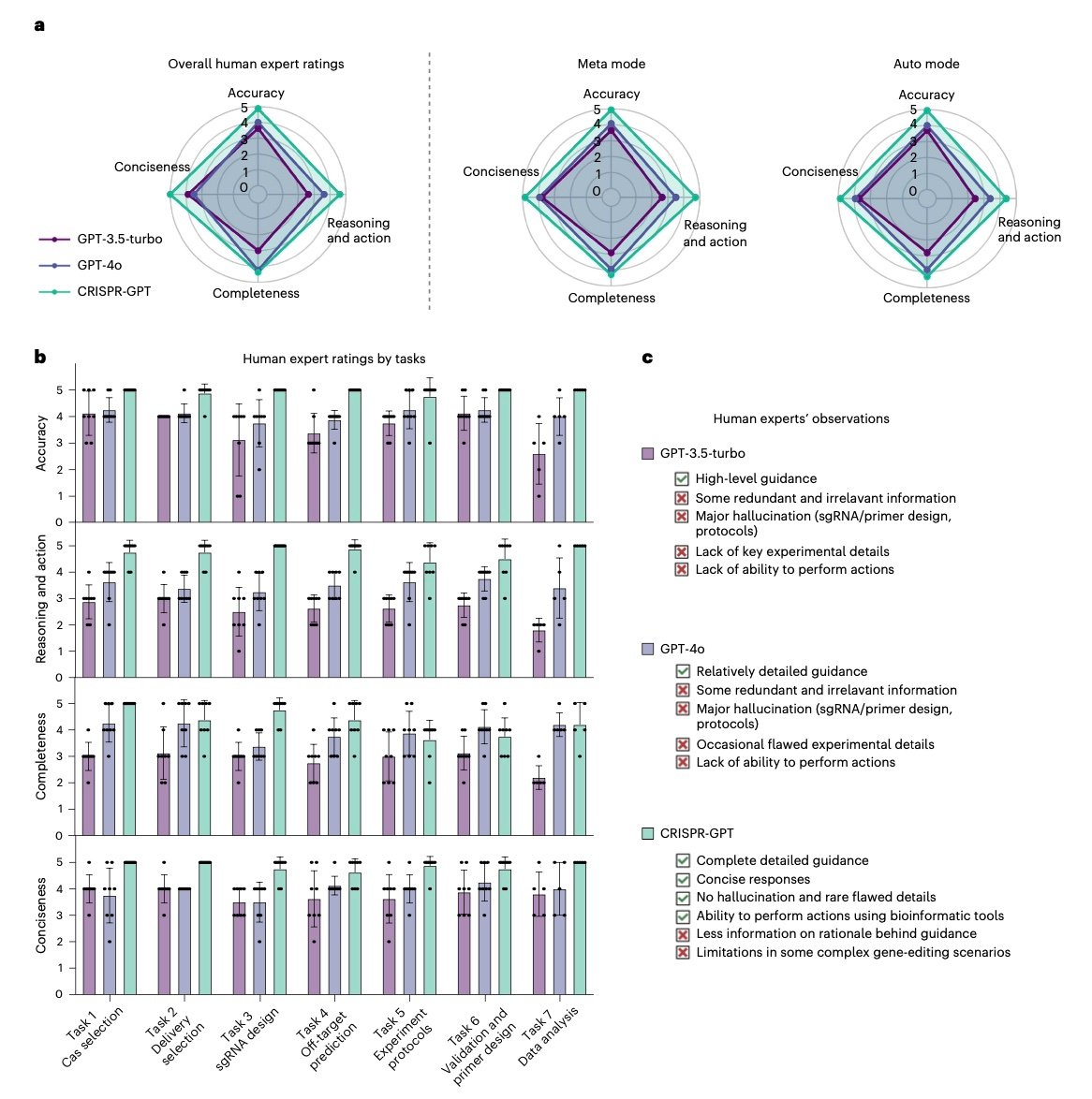
Stanford’s research team, alongside Princeton, UC Berkeley, and Google DeepMind, achieved remarkable results that exceed human expert performance. According to the comprehensive analysis published in GEN Genetic Engineering News, CRISPR-GPT demonstrated 99% planning accuracy across 288 diverse experimental scenarios, while maintaining 80-90% gene editing efficiency in real laboratory conditions.
Additionally, the system successfully automated 22 distinct gene editing tasks, from initial design through final validation. The technical preprint available on bioRxiv details how traditional methods require months of training and often result in lower success rates even among experienced researchers.
The implications become clear when considering recent medical breakthroughs. For instance, a 9-month-old infant with CPS1 deficiency recently received life-saving precision base-editing therapy, costing approximately $800,000—comparable to liver transplant expenses. However, experts predict AI automation could reduce such treatment costs by 30-50% within the next two years.
Breaking Down the Technical Achievement
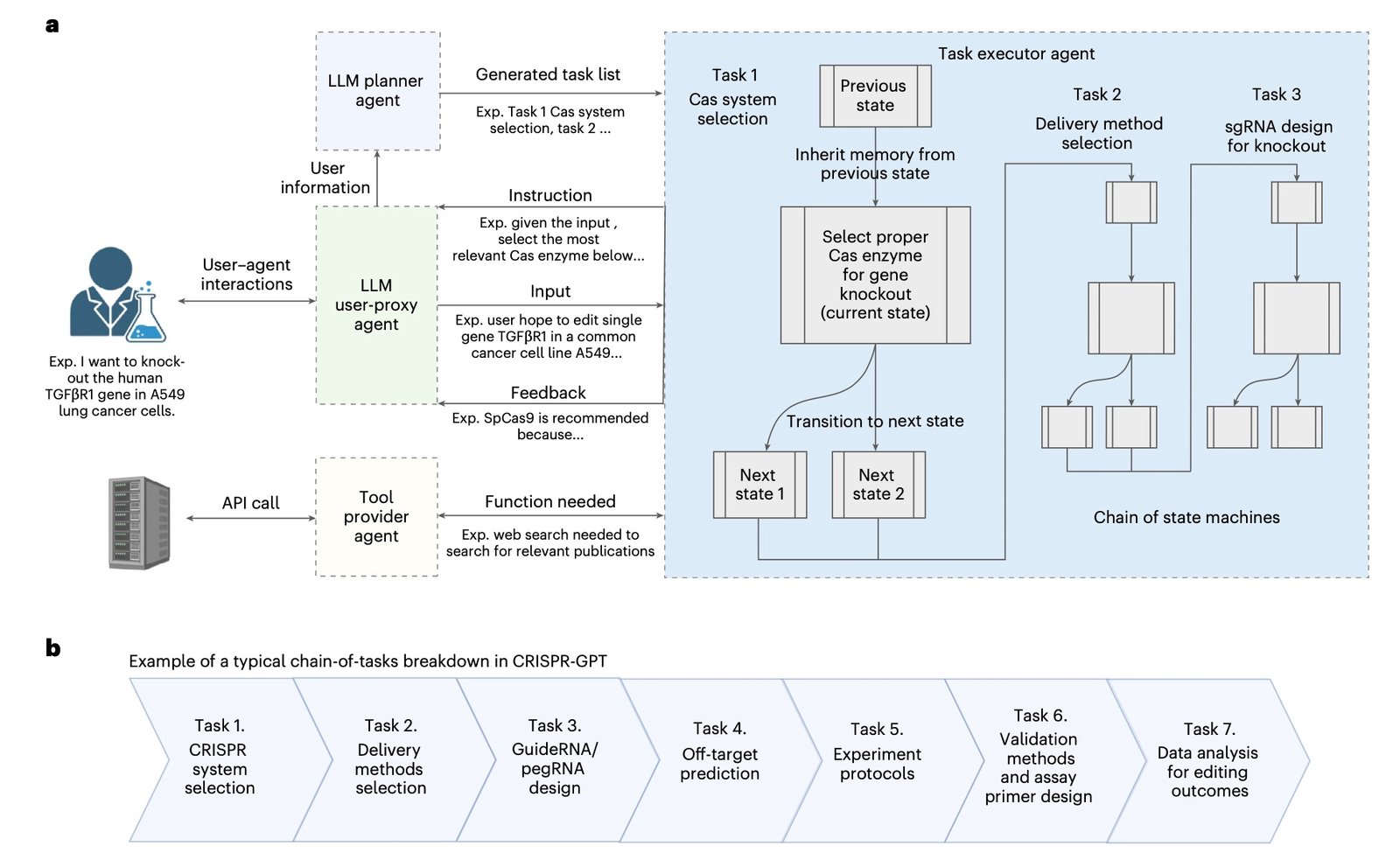
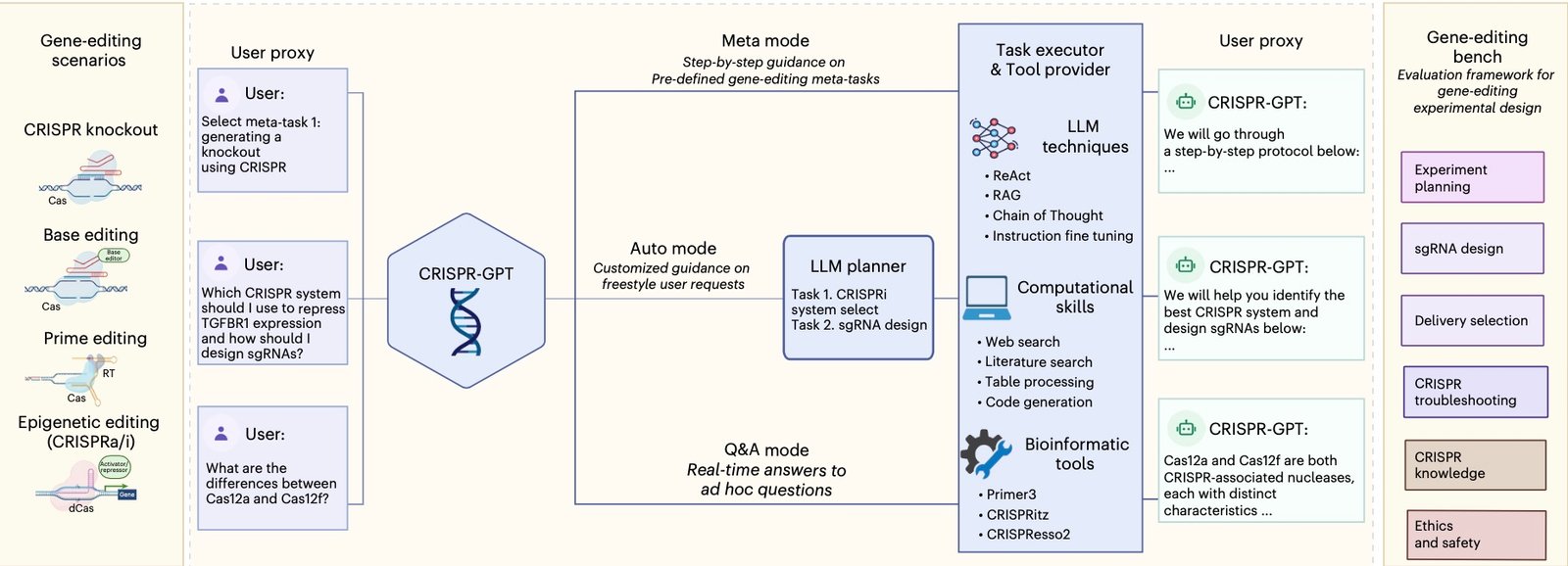
CRISPR-GPT operates through a sophisticated multi-agent architecture comprising four specialized components, as detailed in the full Nature Biomedical Engineering paper. First, the User-proxy agent interfaces with researchers and translates high-level objectives into actionable tasks. Next, the Planner agent decomposes experimental goals into detailed procedural steps.
Furthermore, the Task executor agent handles complex workflows including gRNA design, protocol development, and troubleshooting procedures. Finally, the Tool-provider agent connects to external bioinformatics resources like Primer3 and CRISPResso2, while accessing vast literature databases for optimal delivery method selection.
This breakthrough builds upon similar AI advancements in biotechnology, paralleling how artificial intelligence is accelerating breakthroughs across biological sciences, from cellular engineering to advanced medical applications.
This architecture enables three distinct interaction modes: Meta mode provides step-by-step guidance for beginners, Auto mode generates customized protocols based on user prompts, and Q&A mode offers interactive troubleshooting support.
If you’re interested in creating compelling content about these breakthroughs, Fliki’s AI video platform offers excellent tools for visualizing complex scientific concepts with professional narration and graphics.
America vs China: The New Biotech Cold War
While CRISPR-GPT establishes American leadership in AI-powered genetics, China’s response demonstrates the global stakes involved. As analyzed in Wired’s comprehensive feature on AI-CRISPR convergence, Chinese companies like BDgene, HuidaGene, and Edigene are advancing CRISPR treatments for Duchenne muscular dystrophy and inherited eye diseases.
Moreover, China’s AI models including DeepSeek and Kimi K2 challenge Western dominance in biological applications, similar to how advanced AI systems like OpenAI’s O3 are revolutionizing complex problem-solving across multiple scientific domains. However, CRISPR-GPT’s transparent, peer-reviewed approach contrasts sharply with China’s more secretive development strategies.
The geopolitical implications extend beyond mere scientific competition. Indeed, China enforces strict germline editing bans with prison sentences, while simultaneously supporting non-heritable therapeutic applications. Meanwhile, Western researchers advocate for transparent oversight and safety-first approaches.
Regulatory Frameworks and Global Standards
This divergence in approach creates fascinating regulatory dynamics. For instance, China’s rigid restrictions prevent certain research directions, while potentially accelerating others through centralized coordination. Conversely, America’s open scientific culture enables broader collaboration but requires more complex oversight mechanisms.
CRISPR-GPT’s built-in safety features address these concerns directly. The system flags germline editing queries, prevents biothreat sequence suggestions, and maintains comprehensive audit logs. These safeguards position American AI systems as global standard-bearers for responsible genetic engineering.
For researchers looking to stay current with these rapidly evolving regulations, Coursera’s bioethics courses provide essential background knowledge on navigating complex ethical frameworks in modern biotechnology.
Real-World Medical Impact and Future Applications
The medical implications of CRISPR-GPT extend far beyond laboratory efficiency improvements. Significantly, the system’s ability to reduce experimental timelines from months to hours could accelerate therapeutic development for millions of patients with rare genetic diseases.
Recent clinical successes illustrate this potential. The infant treated for CPS1 deficiency showed marked improvement within months, requiring fewer medications and experiencing reduced metabolic crises. While CRISPR-GPT wasn’t directly involved in this case, it represents exactly the type of therapy that AI automation could make more accessible and affordable.
Furthermore, Stanford’s Qi lab is actively developing CRISPR-Cas13 protocols for neuronal therapy targeting conditions like ALS and spinal muscular atrophy, as reported in Stanford University’s recent announcement. CRISPR-GPT could significantly accelerate hypothesis generation and protocol optimization for these challenging applications.
Want to see how this breakthrough compares to other recent AI advances? Check out our video analysis of the most significant AI developments in 2025.
Democratizing Access to Gene Therapy
Perhaps most importantly, CRISPR-GPT democratizes access to sophisticated genetic engineering capabilities. Previously, only elite research institutions with extensive resources could conduct complex multi-gene editing experiments. Now, smaller academic labs and resource-limited biotech firms can compete on equal footing.
This democratization effect could reduce therapy development costs by 30-50% while expanding the pipeline of potential treatments. Moreover, standardized AI-generated protocols improve reproducibility—a critical factor for regulatory approval processes.
The system’s impact on educational institutions appears equally transformative. Undergraduate students can now gain hands-on gene editing experience without requiring years of specialized training. This educational acceleration could produce a new generation of genetic engineers equipped with both traditional knowledge and AI-assisted capabilities.
For content creators documenting these scientific advances, Pictory’s AI video tools excel at transforming complex research papers into engaging visual narratives that make cutting-edge science accessible to broader audiences.
Technical Deep Dive: How CRISPR-GPT Actually Works
Understanding CRISPR-GPT’s technical architecture reveals why it achieves such remarkable performance improvements. The system’s foundation rests on advanced language models fine-tuned using over 4,000 curated discussion threads spanning more than a decade of expert scientific dialogue.
This training approach enables the AI to internalize scientist-like reasoning patterns, particularly in areas like experimental design, troubleshooting, and safety considerations. Additionally, the multi-agent architecture allows specialized components to handle different aspects of the gene editing workflow simultaneously.
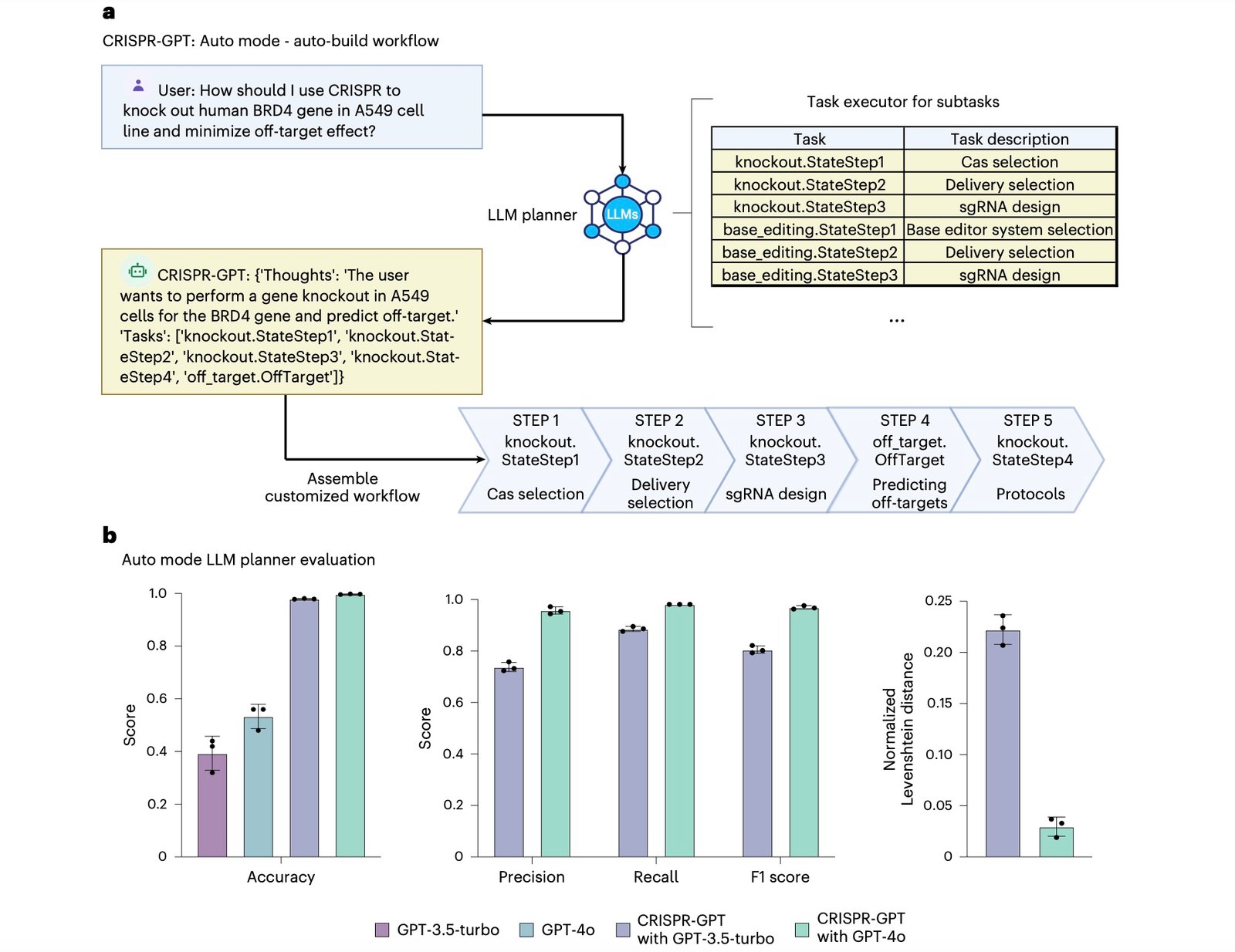
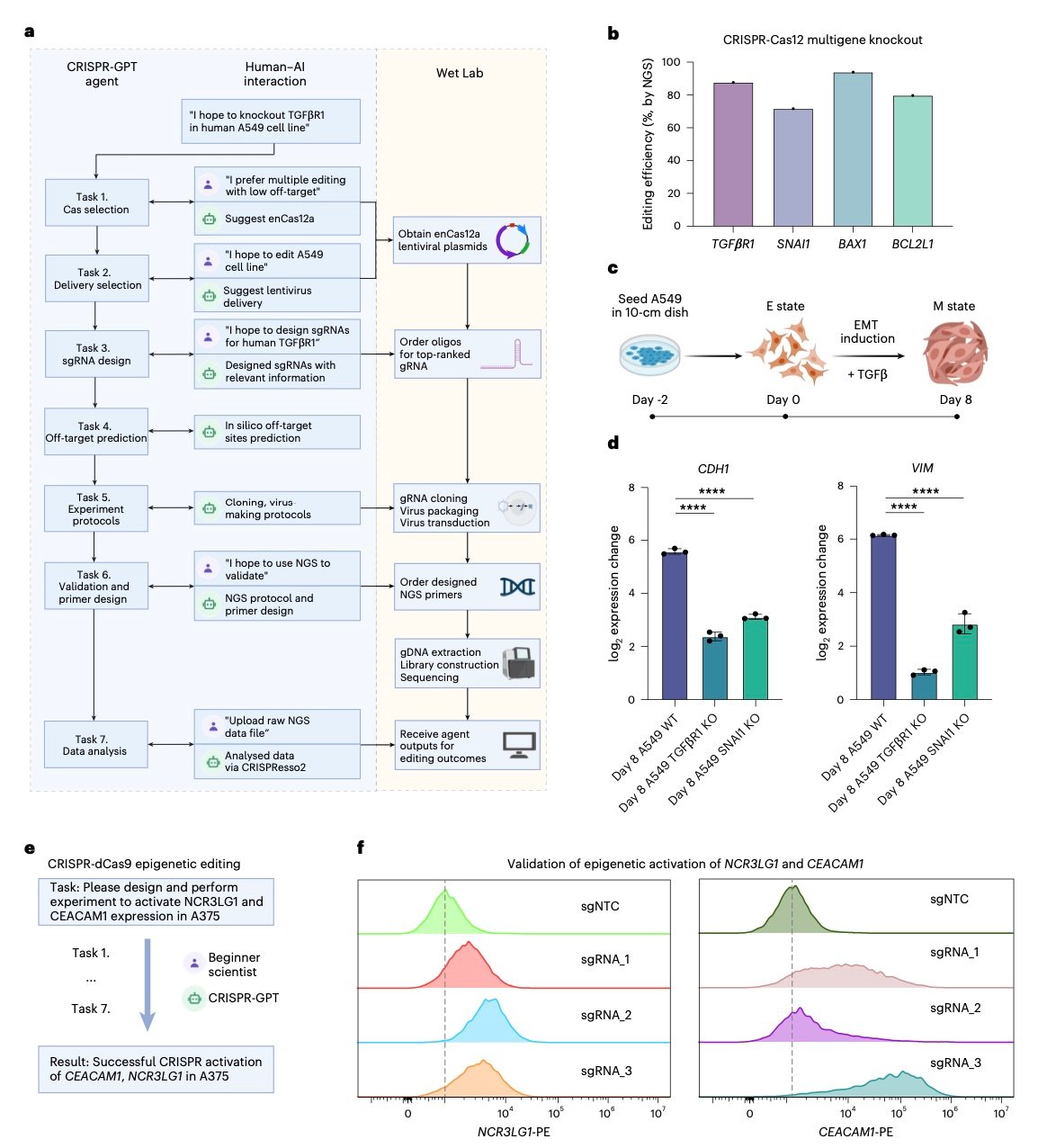
The Planner agent demonstrates particular sophistication in task decomposition. When researchers submit high-level objectives like “knock out the TGFβR1 gene in A549 lung cancer cells,” the system automatically generates comprehensive experimental workflows including Cas system selection, delivery method optimization, gRNA design, off-target analysis, and validation protocols.
Benchmarking Against Human Experts
Rigorous testing across 288 experimental scenarios demonstrates CRISPR-GPT’s superiority over both general-purpose AI models and human experts in specific domains. The system achieved F1 scores exceeding 0.99 in planning accuracy, with precision and recall rates above 99%.
These metrics become even more impressive when considering the complexity of modern gene editing experiments. Traditional approaches often require iterative optimization cycles lasting weeks or months. In contrast, CRISPR-GPT generates optimized protocols in minutes while incorporating safety checks and regulatory compliance considerations.
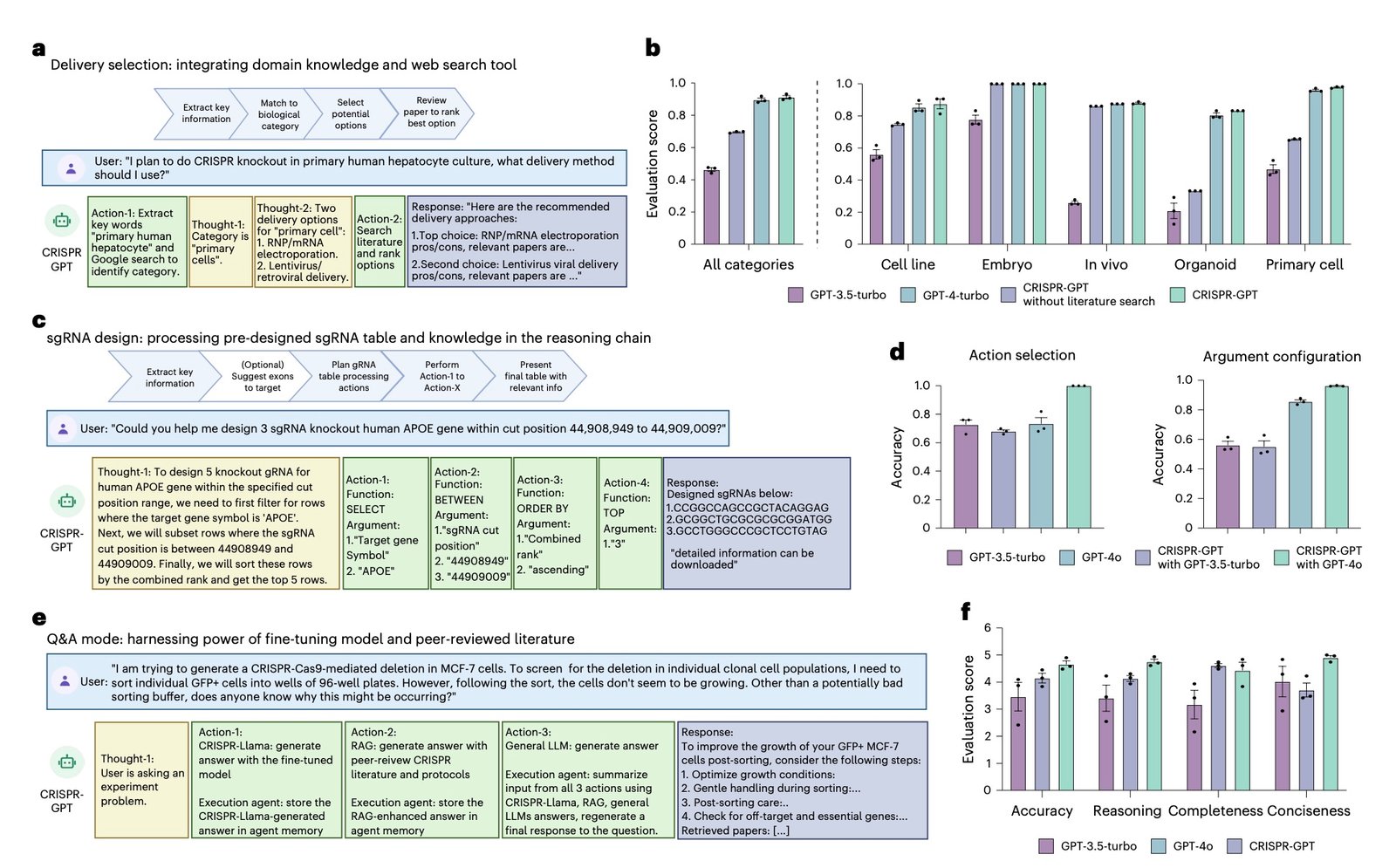
The system’s gRNA design capabilities particularly impress experts. Unlike existing tools that simply rank candidates by computational scores, CRISPR-GPT considers functional context, exon importance, and experimental objectives. This approach led to successful gene knockouts targeting functionally critical protein domains—outcomes that previous automated tools frequently missed.
For businesses looking to leverage these advances in their own content marketing, Synthesia’s AI avatar technology enables companies to create professional scientific presentations without requiring on-camera talent or expensive video production.
Ethical Considerations and Safety Safeguards
While CRISPR-GPT’s capabilities inspire excitement, they also raise important ethical questions about democratizing powerful genetic engineering tools. Critics worry that automated protocol design could enable less qualified users to inadvertently create off-target effects or even dual-use applications.
The development team addressed these concerns through multiple safety layers. First, the system includes built-in compliance checking that flags germline editing queries and prevents biothreat sequence suggestions. Second, comprehensive audit logging ensures all system interactions remain traceable for regulatory review.
Additionally, CRISPR-GPT’s training data underwent careful curation to exclude potentially harmful information while preserving scientific accuracy. The system actively promotes safety-first approaches and encourages users to follow established ethical guidelines.
Privacy and Data Security Concerns
Genetic information privacy represents another critical consideration. CRISPR-GPT must handle potentially sensitive patient data while maintaining strict confidentiality standards. The system implements HIPAA-compliant data governance procedures and includes filters to prevent inadvertent sharing of identifiable genetic sequences.
Moreover, the system’s transparency requirements balance between scientific openness and security concerns. While the underlying model training remains partially confidential, the core methodologies and safety procedures are fully documented in peer-reviewed publications.
These ethical frameworks become increasingly important as gene editing capabilities expand. The establishment of global standards for AI-driven biological agents will likely require international cooperation and ongoing regulatory development.
For organizations needing to communicate complex ethical frameworks to diverse audiences, Beehiiv’s newsletter platform offers excellent tools for building engaged subscriber communities around specialized scientific topics.
Economic Impact and Market Transformation
CRISPR-GPT’s economic implications extend far beyond immediate research efficiency gains. Analysts predict the system could catalyze a fundamental transformation in biotechnology market dynamics, particularly in therapeutic development and manufacturing costs.
Current gene therapy development typically requires $1-2 billion investments over 10-15 year timelines. However, AI automation could compress these timelines to 3-5 years while reducing costs by 30-50%. This transformation would make previously uneconomical rare disease treatments financially viable.
Furthermore, the democratization effect could spawn thousands of new biotech startups focusing on specialized therapeutic niches. Previously, only well-funded companies could afford the expertise and infrastructure necessary for complex gene editing projects. Now, smaller teams with innovative ideas can compete effectively using AI-powered tools.
Investment and Funding Implications
Venture capital firms are already recognizing these shifts. Investment in AI-biotech convergence companies increased 340% in 2024, with CRISPR-GPT’s success likely to accelerate this trend further. Companies demonstrating successful integration of AI design tools with therapeutic development are commanding premium valuations.
Moreover, pharmaceutical giants are reassessing their internal R&D strategies. Many are establishing partnerships with AI-focused biotechs rather than developing comparable capabilities in-house. This trend could reshape industry consolidation patterns over the next decade.
The educational implications also create economic opportunities. Universities incorporating CRISPR-GPT into their curricula can produce graduates with immediately applicable skills, potentially commanding higher starting salaries in an increasingly competitive job market.
For individuals looking to position themselves advantageously in this evolving landscape, Surfshark VPN provides secure access to global research databases and international collaboration platforms essential for staying current with rapid developments.
Global Competition and Strategic Implications
CRISPR-GPT’s emergence intensifies international competition in biotechnology leadership. While American institutions currently maintain technical advantages through transparent research and robust peer review processes, other nations are rapidly developing competing capabilities.
China’s biotech initiatives demonstrate particular ambition. The country’s centralized research coordination enables rapid resource deployment, while companies like BDgene advance clinical applications at unprecedented speeds. However, regulatory restrictions on certain research directions may limit long-term competitiveness.
European Union approaches emphasize ethical frameworks and regulatory harmonization. While this creates some development delays, it may ultimately produce more globally acceptable standards for AI-assisted genetic engineering. The EU’s proposed AI regulations specifically address biotechnology applications and could influence global governance frameworks.
Implications for Developing Nations
CRISPR-GPT’s democratization effects could prove particularly transformative for developing nations. Countries with limited traditional biotechnology infrastructure can now access world-class genetic engineering capabilities through AI systems rather than expensive human expertise.
This leveling effect might enable breakthrough treatments for region-specific genetic diseases that previously received little research attention. Furthermore, reduced development costs could make treatments affordable in markets with lower purchasing power.
However, regulatory capacity limitations in some developing nations raise safety concerns. International cooperation on AI governance frameworks becomes essential to prevent inadvertent risks while maximizing therapeutic benefits.
The strategic implications extend to national security considerations. Countries leading in AI-biotech convergence may gain significant advantages in pharmaceutical independence, agricultural innovation, and even biological defense capabilities.
Future Timeline and Predictions
Looking ahead to 2026-2030, CRISPR-GPT’s integration with laboratory automation systems promises to create fully autonomous experimental platforms. Leading institutions like Stanford and UC Berkeley are already developing robotic systems capable of executing AI-generated protocols without human intervention.
Clinical applications appear likely by late 2025 or early 2026, beginning with rare genetic diseases where regulatory pathways are most established. The infant CPS1 case demonstrates regulatory willingness to approve innovative approaches for life-threatening conditions with limited alternatives.
Cost reductions seem inevitable. Current projections suggest gene therapy expenses dropping from $1 million per patient to under $500,000 by 2026, with further decreases likely as automation scales. These improvements could make genetic treatments accessible to middle-class patients rather than only the ultra-wealthy.
Technology Integration Roadmap
Short-term developments (2025-2026) will likely focus on clinical validation and regulatory integration. Mid-term advances (2026-2028) should emphasize automation integration and cost optimization. Long-term evolution (2028-2030) may include artificial general intelligence applications enabling truly autonomous therapeutic design.
The convergence with other emerging technologies creates additional possibilities. Integration with quantum computing could enable more sophisticated molecular modeling, while advances in delivery mechanisms might expand treatable conditions dramatically.
Educational transformation appears equally certain. Within five years, genetic engineering education will likely require AI literacy as a core competency. Students entering the field must understand both traditional molecular biology and AI-assisted design principles.
However, challenges remain significant. Regulatory frameworks must evolve rapidly to keep pace with technological capabilities. International coordination becomes essential to prevent regulatory arbitrage while maintaining safety standards.
Conclusion: The Dawn of Automated Medicine
CRISPR-GPT represents more than just another scientific breakthrough—it signals the beginning of truly automated medicine where artificial intelligence designs personalized treatments with unprecedented speed and accuracy. The system’s 99% planning accuracy and ability to transform novices into successful genetic engineers demonstrates AI’s potential to democratize the most sophisticated medical technologies.
The complete research findings, including detailed methodology and experimental results, are available in the peer-reviewed Nature Biomedical Engineering publication, establishing this work as a cornerstone reference for future AI-driven biotechnology developments.
The geopolitical implications are equally profound. America’s leadership in transparent, safety-focused AI development contrasts sharply with other nations’ approaches, potentially establishing global standards for responsible genetic engineering automation. As China and other competitors develop rival systems, maintaining this leadership will require continued investment in both technical capabilities and ethical frameworks.
Most importantly, CRISPR-GPT offers hope for millions of patients with genetic diseases who previously had no treatment options. By reducing development timelines from years to months and costs from millions to hundreds of thousands of dollars, AI automation could finally make genetic medicine accessible to ordinary families rather than just the ultra-wealthy.
The revolution is just beginning. Within five years, AI-designed gene therapies may become as routine as traditional pharmaceutical prescriptions, transforming medicine from an art based on statistical averages to a precision science tailored to individual genetic profiles.
As we stand at this technological inflection point, one thing remains certain: the future of medicine will be written by artificial intelligence, and CRISPR-GPT has just authored the opening chapter.
Frequently Asked Questions (FAQs)
What is CRISPR GPT?
CRISPR-GPT is a revolutionary AI system developed by Stanford University, Princeton, UC Berkeley, and Google DeepMind that automates the entire gene editing workflow. Unlike traditional CRISPR technology that requires extensive human expertise, CRISPR-GPT uses advanced language models and multi-agent architecture to guide researchers through complex genetic engineering experiments with 99% planning accuracy. The system can design guide RNAs, select delivery methods, optimize protocols, and analyze results automatically.
What is Crispr gpt llm agent?
The CRISPR-GPT LLM agent is a sophisticated multi-component AI system consisting of four specialized agents: a User-proxy agent that interfaces with researchers, a Planner agent that decomposes experimental objectives, a Task executor agent that handles workflows like gRNA design and protocol development, and a Tool-provider agent that connects to external bioinformatics resources. This agent-based architecture enables the system to reason through complex genetic engineering problems and provide step-by-step guidance to users with varying levels of expertise.
What is the biggest concern with CRISPR?
The biggest concerns with CRISPR technology include off-target effects (unintended genetic modifications), potential germline editing that could affect future generations, ethical implications of “designer babies,” and the democratization of genetic engineering tools that could be misused. With AI automation like CRISPR-GPT, additional concerns arise about ensuring proper oversight, preventing misuse by unqualified users, and maintaining human control over critical genetic decisions. However, CRISPR-GPT includes built-in safety mechanisms to flag risky applications and prevent unauthorized uses.
Did CRISPR win the Nobel Prize?
Yes, CRISPR technology won the Nobel Prize in Chemistry in 2020. The award was given to Jennifer Doudna (UC Berkeley) and Emmanuelle Charpentier (Max Planck Institute) for developing the CRISPR-Cas9 genetic scissors. Their groundbreaking work laid the foundation for modern gene editing, which AI systems like CRISPR-GPT now build upon to automate and democratize genetic engineering capabilities.
What are some negative things about CRISPR?
Negative aspects of CRISPR include potential off-target effects that could cause unintended genetic damage, high costs that limit accessibility, technical complexity requiring specialized expertise, regulatory uncertainty, and ethical concerns about human enhancement and genetic inequality. Additionally, there are risks of immune reactions to CRISPR components, challenges in delivering treatments to specific tissues, and potential long-term consequences that aren’t yet fully understood. CRISPR-GPT addresses some of these issues by improving accuracy and reducing costs, but many concerns remain.
What diseases could CRISPR get rid of in the future?
CRISPR technology, especially when enhanced by AI systems like CRISPR-GPT, could potentially treat or cure numerous genetic diseases including sickle cell disease, beta-thalassemia, Duchenne muscular dystrophy, cystic fibrosis, Huntington’s disease, various inherited eye diseases, some forms of cancer, HIV infections, and rare metabolic disorders like CPS1 deficiency. The automation provided by CRISPR-GPT could accelerate the development of personalized treatments for thousands of genetic conditions by reducing development timelines from years to months.
How accurate is CRISPR-GPT compared to human experts?
CRISPR-GPT demonstrates superior performance compared to human experts in many areas, achieving 99% planning accuracy across 288 experimental scenarios and 80-90% gene editing efficiency in laboratory tests. The system outperforms general AI models like GPT-4 and GPT-3.5 in domain-specific gene editing tasks, while enabling complete novices to achieve successful results that typically require months of specialized training. However, human oversight remains essential for complex cases and ethical decision-making.
When will CRISPR-GPT be available for clinical use?
While CRISPR-GPT was published in Nature Biomedical Engineering in July 2025, clinical applications are expected to begin with rare genetic diseases in late 2025 or early 2026. The system is currently validated for laboratory use and research applications. Full integration into clinical practice will require regulatory approval from agencies like the FDA, which typically takes 1-3 years depending on the specific application and safety requirements.
How much does CRISPR-GPT cost?
Current gene therapy development costs range from $1-2 million per treatment, but experts predict CRISPR-GPT’s automation could reduce these costs by 30-50%, bringing treatments down to approximately $500,000 per patient by 2026. The democratization effect of AI automation should make genetic engineering accessible to smaller laboratories and research institutions that previously couldn’t afford the necessary expertise and infrastructure.
Is CRISPR-GPT safe?
CRISPR-GPT incorporates multiple safety mechanisms including built-in compliance checking that flags germline editing queries, prevention of biothreat sequence suggestions, comprehensive audit logging, and HIPAA-compliant data governance. The system actively promotes safety-first approaches and includes filters to prevent sharing of identifiable genetic sequences. However, like all powerful technologies, it requires responsible use and appropriate regulatory oversight.
Can CRISPR-GPT work on all types of genetic diseases?
CRISPR-GPT supports four major gene editing modalities: knockout, base editing, prime editing, and epigenetic editing (CRISPRa/i), making it versatile for many types of genetic conditions. However, its effectiveness depends on factors like the specific genetic mechanism involved, tissue accessibility for treatment delivery, and the current state of CRISPR technology for particular applications. The system is most effective for single-gene disorders and may have limitations with complex multi-gene conditions.
Ready to stay ahead of AI-powered biotechnology trends? Subscribe to our newsletter for the latest breakthrough analyses and strategic insights.

Pingback: The Most Mind-Blowing Tech Predictions for 2030
Pingback: Magnetic Micro-Robots Target Kidney Stones - Waterloo 2025