Bottom Line Up Front: Chinese surgeons have achieved the impossible—successfully transplanting a genetically modified pig lung into a human patient and keeping it functional for 9 days without catastrophic rejection. This represents the most challenging xenotransplantation milestone ever achieved and marks a turning point in our battle against the global organ shortage crisis that kills 17 people daily.
The Breakthrough That Rewrote Medical History
On August 25, 2025, the pages of Nature Medicine carried a study that would fundamentally alter the trajectory of transplant medicine forever. Dr. Jianxing He and his team at Guangzhou Medical University’s First Affiliated Hospital had accomplished what generations of scientists deemed nearly impossible: they had successfully transplanted a pig lung into a human being and sustained its function for over a week.
The achievement sent shockwaves through the global medical community, not merely because it succeeded, but because it conquered what many considered the most formidable challenge in xenotransplantation—the lung. While pig kidneys and hearts have made headlines in recent years, the lung represents the Mount Everest of cross-species organ transplantation, a summit that had remained unconquered despite decades of scientific assault.
The recipient was a 39-year-old man who had suffered catastrophic brain hemorrhage and was declared brain dead. His family, in an act of profound generosity, consented to the experimental procedure that would provide crucial data for future patients facing certain death on organ waiting lists. What followed over the next 216 hours would challenge everything we thought we knew about the limits of xenotransplantation.
The pig lung didn’t just survive—it thrived. It produced oxygen, removed carbon dioxide, and maintained the delicate balance of respiratory physiology that keeps human life possible. For nine days, a pig organ served as the bridge between life and death in a human chest, proving that the biological barriers between species, while formidable, are not insurmountable.
The Anatomy of Medical Impossibility: Why Lungs Are Different
To understand the magnitude of this achievement, one must first grasp why lung xenotransplantation has remained the final frontier. The lung is not merely another organ—it’s a biological interface between the internal human environment and the external world, a complexity that makes it uniquely vulnerable to rejection and failure.
The Exposure Challenge Unlike kidneys, hearts, or livers that operate in the sterile confines of the body cavity, lungs are in constant contact with the outside world. Every breath introduces potential pathogens, allergens, and inflammatory agents directly into the organ system. This creates a state of perpetual immune vigilance that makes the lung hypersensitive to any foreign material, including transplanted tissue.
Surface Area Supremacy The human lung contains approximately 300 million alveoli, creating a surface area roughly equivalent to a tennis court—all packed into the space of two footballs. This massive surface area, while essential for gas exchange, provides countless opportunities for immune system activation. Each microscopic air sac represents a potential battleground where human immune cells can encounter pig tissue and mount an attack.
Vascular Complexity The pulmonary circulation system processes the entire cardiac output every minute—roughly 5 liters of blood flowing through an intricate network of capillaries thinner than human hair. Any incompatibility between pig and human blood systems becomes immediately apparent, as there’s no time for gradual adaptation or immune tolerance development.
The Inflammatory Cascade Lungs are particularly susceptible to primary graft dysfunction (PGD)—a condition where transplanted lungs develop severe inflammation and fluid accumulation within hours of transplantation. Even in human-to-human lung transplants, PGD occurs in 10-25% of cases and represents the leading cause of early graft failure. In xenotransplantation, this risk is exponentially higher due to species-specific inflammatory responses.
These factors collectively explain why lung xenotransplantation has lagged behind other organs. Previous attempts have failed within hours, victims of hyperacute rejection—the immune system’s immediate, violent response to foreign tissue that results in massive inflammation, blood clotting, and organ failure.
The Genetic Engineering Revolution: Creating the Perfect Pig
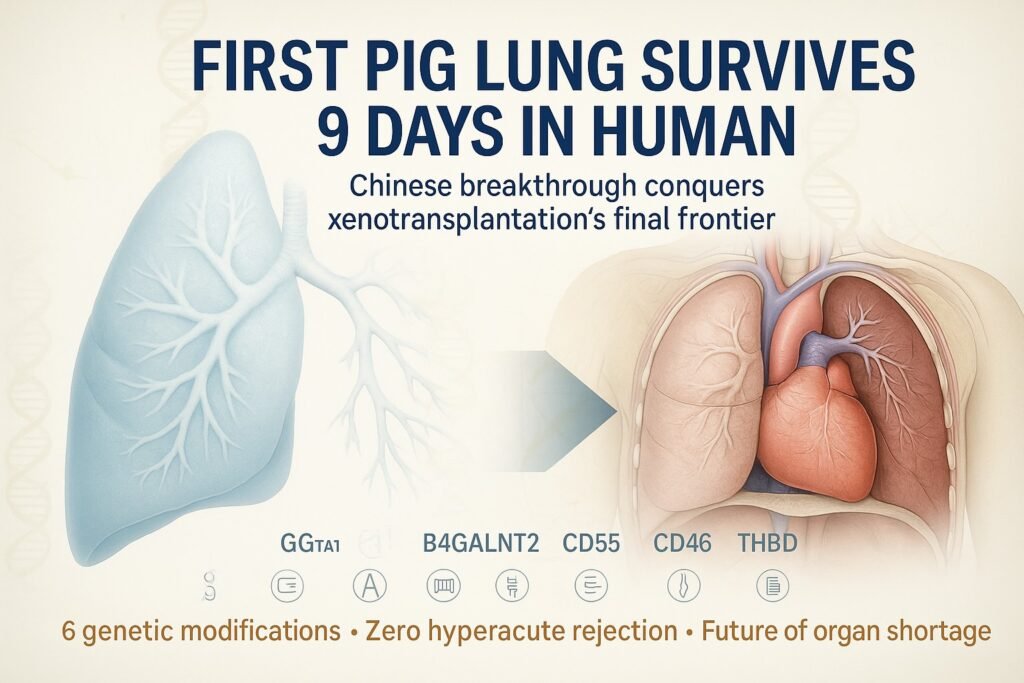
The breakthrough in Guangzhou didn’t happen overnight. It represented the culmination of years of genetic engineering work aimed at creating pigs whose organs would be compatible with human physiology. The pig used in this historic transplant carried six precise genetic modifications, each addressing a specific biological incompatibility between pigs and humans.
The Triple Knockout Strategy The first line of genetic defense involved removing three pig genes responsible for producing molecules that immediately trigger human immune responses:
GGTA1 Elimination: This gene produces α-galactosyl transferase, an enzyme that creates α-Gal sugar molecules on pig cell surfaces. Humans naturally produce antibodies against α-Gal, leading to immediate organ rejection. By knocking out GGTA1, the modified pigs no longer express these problematic sugars.
CMAH Deletion: The CMAH gene enables pigs to produce Neu5Gc, a sialic acid variant that humans recognize as foreign. Human anti-Neu5Gc antibodies can trigger complement activation and organ rejection. Eliminating this gene removes another major rejection trigger.
B4GALNT2 Knockout: This gene produces enzymes responsible for creating additional sugar antigens (including the Sd(a) blood group antigen) that can provoke human immune responses. Its removal further reduces the immunological distance between pig and human tissue.
The Human Gene Integration Beyond removing problematic pig genes, the scientists added three human genes to make the pig organs more compatible with human physiology:
CD55 (Decay Accelerating Factor): This human protein regulates complement activation, preventing the cascade of immune responses that can destroy transplanted organs. By expressing human CD55, the pig lung could better control human complement activation on its surface.
CD46 (Membrane Cofactor Protein): Working alongside CD55, CD46 provides additional complement regulation, creating multiple layers of protection against immune attack. These proteins essentially teach the human immune system that the pig tissue is safe.
THBD (Thrombomodulin): Perhaps most importantly, thrombomodulin addresses blood clotting incompatibilities between species. Pig and human coagulation systems have subtle but critical differences that can lead to dangerous blood clots. Human thrombomodulin helps regulate clotting on the pig lung surface, preventing thrombotic complications.
This “6GE” configuration represents a masterpiece of genetic engineering, each modification addressing a specific failure mode identified in previous xenotransplantation attempts. The precision gene editing that enabled the six genetic modifications in the Guangzhou pig would have been impossible just a decade ago. CRISPR-Cas9 technology allows scientists to make precise changes to DNA sequences, removing problematic genes and inserting beneficial ones with unprecedented accuracy. This revolutionary approach to genetic modification is transforming medicine in ways we predicted, with AI-guided gene editing becoming reality faster than expected.
Nine Days That Changed Everything: A Detailed Chronicle
The transplantation procedure began on what would become Day Zero in transplant medicine history. The surgical team, led by Dr. Jianxing He, faced the daunting task of removing the patient’s left lung and replacing it with the genetically modified pig organ while maintaining respiratory function through the right lung.
Day Zero: The Surgical Marvel The operation itself represented a technical tour de force. Unlike kidney transplants, where the recipient’s original kidneys often remain in place, lung transplantation requires precise removal of the diseased organ and meticulous connection of the replacement’s blood vessels and airways. The fact that they were connecting pig tissue to human anatomy added layers of complexity that had never been attempted at this scale.
The immediate post-operative period held everyone’s breath—literally and figuratively. Would the pig lung integrate with human circulation? Would hyperacute rejection occur within minutes as it had in previous attempts? The answers came quickly: blood flow was established, oxygen exchange began, and the dreaded immediate rejection did not materialize.
Day One: The Primary Challenge Twenty-four hours post-transplant, the first major complication appeared: severe pulmonary edema, or fluid accumulation in the lung tissue. This condition, known as primary graft dysfunction (PGD), represents one of the most feared complications in lung transplantation. The transplanted lung became swollen and inflamed, its ability to exchange oxygen and carbon dioxide severely compromised.
In human-to-human transplants, severe PGD can be fatal and often indicates that the organ will never function properly. The medical team faced a critical decision point: was this the beginning of the end, or could the pig lung overcome this early setback? They chose to continue aggressive supportive care, maintaining the patient on mechanical ventilation and administering anti-inflammatory treatments.
Days Two through Five: The Waiting Game The following days represented a delicate balance between hope and concern. The pig lung’s function fluctuated, showing moments of improvement followed by periods of declining performance. Medical teams monitored dozens of parameters hourly: blood oxygen levels, carbon dioxide elimination, lung pressures, inflammatory markers, and signs of rejection.
Importantly, hyperacute rejection—the immediate, catastrophic immune response that destroys xenotransplanted organs within hours—never occurred. This absence represented a vindication of the genetic engineering approach. The human immune system, while not embracing the pig lung, was not mounting the violent, immediate attack that had doomed previous attempts.
Day Three: The First Rejection Episode The relative calm was broken on Day Three when blood tests revealed rising levels of antibodies directed against the pig lung tissue. This antibody-mediated rejection (AMR) represents a more delayed but equally dangerous immune response where the body produces specific antibodies that attack the transplanted organ.
The medical team responded with intensified immunosuppression, administering additional doses of anti-rejection medications. This represented a critical test: could modern immunosuppressive protocols control pig-specific rejection, or were the species differences too great to overcome?
Day Six: Crisis and Recovery A second wave of antibody-mediated rejection struck on Day Six, more severe than the first episode. Antibody levels spiked, and the lung’s function deteriorated measurably. This represented the most dangerous moment of the entire experiment—the point where many expected the pig lung to fail permanently.
The medical team’s response was swift and comprehensive. They administered plasmapheresis to remove circulating antibodies, intensified immunosuppressive therapy, and provided maximum mechanical ventilation support. The next twenty-four hours would determine whether this extraordinary experiment would end in failure or continue its historic run.
Day Nine: The Remarkable Recovery Against all expectations, the pig lung began to recover. Antibody levels decreased, inflammatory markers improved, and most importantly, gas exchange function stabilized. By Day Nine, the organ was not only surviving but showing signs of adaptation to its human environment.
The recovery wasn’t complete—the lung still showed signs of stress and required significant medical support. However, the fact that it could recover from severe rejection episodes demonstrated a level of resilience that exceeded all previous xenotransplantation attempts.
The Final Assessment When the experiment concluded after 216 hours (nine days), the pig lung remained viable and functional. It had survived hyperacute rejection, overcome primary graft dysfunction, and recovered from multiple rejection episodes. While not perfect, it had proven that pig lungs could support human life for extended periods—a feat many thought impossible just months earlier.
The Immunological War: Understanding What Went Right
The success of the Guangzhou experiment wasn’t just about surgical skill or genetic engineering—it required a sophisticated understanding of immunology and the deployment of an unprecedented immunosuppressive regimen. The patient received a combination of eight different medications, each targeting a specific aspect of the immune response.
The Antibody Arsenal The immunosuppressive protocol began with antithymocyte globulin (ATG), a powerful medication that depletes T-cells—the immune system’s primary attack force. This was followed by basiliximab, which blocks IL-2 receptors and prevents T-cell activation. Rituximab targeted B-cells, the antibody-producing factories that were responsible for the rejection episodes on Days Three and Six.
Complement Control Eculizumab, a groundbreaking complement inhibitor, prevented the cascade of immune reactions that can destroy transplanted organs. This medication, originally developed for rare blood disorders, has become a crucial tool in xenotransplantation research.
Advanced Immunomodulation Tofacitinib, a JAK inhibitor originally developed for rheumatoid arthritis, provided additional immune system modulation. Combined with traditional transplant medications like tacrolimus and mycophenolate, this created a comprehensive shield against rejection.
The Steroid Bridge Corticosteroids provided broad-spectrum anti-inflammatory effects, helping control the severe inflammation that characterized the early post-transplant period. The gradual reduction of steroid doses tested whether the pig lung could maintain function with less intensive immunosuppression.
This eight-drug regimen represents the most intensive immunosuppressive protocol ever attempted in xenotransplantation. While too toxic for long-term use in living patients, it provided the proof-of-concept that pig organ rejection could be controlled with appropriate medical intervention.
Global Context: The Organ Crisis That Demands Solutions
The success in Guangzhou couldn’t come at a more critical time. The global organ shortage has reached crisis proportions, with the gap between supply and demand widening each year despite improvements in organ procurement and allocation.
The Devastating Numbers Worldwide, over 173,000 organ transplants were performed in 2024, representing a significant increase from previous years but still falling far short of need. According to the World Health Organization’s Global Observatory on Donation and Transplantation, of these, only 8,236 were lung transplants—a tragically small number given the thousands waiting for these life-saving organs.
In Europe, the statistics paint a grim picture: 3,926 patients currently wait for lung transplants, while 216 died on waiting lists in 2024 alone. The European average waiting time for lungs exceeds 18 months, and many patients deteriorate beyond transplant candidacy while waiting.
The United States, despite having one of the world’s most advanced transplant systems, performed over 48,000 organ transplants in 2024, with lung transplants increasing 10.4% year-over-year. However, this growth still cannot meet demand, and approximately 17 people die daily waiting for organ transplants of all types.
The Mathematics of Scarcity The fundamental problem is mathematical: organ supply is constrained by the relatively small number of brain-dead donors who die in circumstances that preserve organ viability. Despite decades of public awareness campaigns, donor rates have plateaued in most developed countries, unable to keep pace with aging populations and increasing rates of end-stage organ disease.
Lung transplantation faces additional challenges. Unlike kidneys, where living donation is possible, or hearts, where mechanical devices can provide temporary support, lung failure offers few alternatives. Mechanical ventilation can sustain life temporarily, but there’s no equivalent to dialysis for lung function. Patients in end-stage lung disease face a binary choice: receive a transplant or die.
The Economic Burden The organ shortage creates enormous economic costs beyond the human suffering. End-stage organ disease patients consume disproportionate healthcare resources while waiting for transplants. In the US alone, the annual cost of caring for patients with end-stage renal disease exceeds $50 billion, much of which could be avoided with adequate organ availability.
Xenotransplantation offers the theoretical possibility of unlimited organ supply. Pigs can be bred in controlled environments, genetically modified for compatibility, and harvested when organs reach optimal condition. This could transform transplant medicine from a scarcity-based system to an abundance-based one, fundamentally altering the economics and ethics of organ allocation.
The Path Forward: Challenges That Remain Formidable
Despite the success in Guangzhou, significant obstacles remain before pig lungs could be used routinely in living patients. Each challenge represents years of additional research and development, requiring sustained scientific effort and substantial investment.
Genetic Optimization: Beyond the Current Model The six genetic modifications used in Guangzhou represent first-generation engineering. Future pigs will likely require 10-15 genetic changes to achieve optimal compatibility. Scientists are investigating additional modifications including:
Enhanced Coagulation Control: Adding human EPCR (endothelial protein C receptor) and CD39 genes to further reduce thrombotic risk. Pig and human coagulation systems have subtle differences that can lead to dangerous blood clots even with current modifications.
Advanced Complement Regulation: Incorporating additional complement control proteins like CD35 and factor H to provide even more robust protection against immune attack.
Inflammatory Response Modification: Engineering pigs to express human cytokine receptors and anti-inflammatory molecules to better control the inflammatory response that contributes to primary graft dysfunction.
PERV Inactivation: Eliminating porcine endogenous retroviruses (PERVs) that could potentially infect human recipients. While no PERV transmission has been documented in clinical xenotransplantation, regulatory agencies require comprehensive virus elimination before approving trials in living patients.
Immunosuppression Revolution: Reducing Toxicity The eight-drug regimen used in Guangzhou, while effective, is far too toxic for use in living patients. Future protocols will focus on more targeted interventions:
Costimulation Blockade: Anti-CD40 antibodies and other costimulation blockers could provide more specific immune control with less toxicity than current broad-spectrum immunosuppression.
Tolerance Induction: Strategies to induce immune tolerance, where the recipient’s immune system learns to accept the pig organ as “self,” could eliminate the need for lifelong immunosuppression.
Localized Immunosuppression: Techniques to deliver immunosuppressive agents directly to the transplanted organ could minimize systemic side effects while maintaining local immune control.
Infection Control: The PERV Problem Porcine endogenous retroviruses represent perhaps the most significant regulatory hurdle for xenotransplantation. These viruses are integrated into pig DNA and cannot be eliminated through conventional screening. While they can infect human cells in laboratory conditions, no transmission has been documented in xenotransplantation recipients.
Nevertheless, regulatory agencies require comprehensive virus elimination strategies. According to International Xenotransplantation Association guidelines, companies like eGenesis have developed PERV-inactivated pigs using CRISPR technology, but these animals are still undergoing safety testing. The FDA has maintained cautious optimism about xenotransplantation progress while emphasizing safety requirements. Dr. Peter Marks, Director of the Center for Biologics Evaluation and Research, noted that “while these results are encouraging, they represent early proof-of-concept rather than clinical readiness. We will require extensive additional data on safety, efficacy, and infection risk before considering approval for living patient trials.”
The regulatory framework for xenotransplantation requires lifetime monitoring of recipients, with biological samples preserved for 50 years to track potential virus transmission. This unprecedented level of surveillance reflects the unknown long-term risks of cross-species transplantation.
Organ Preservation and Transport Current organ preservation techniques, developed for human-to-human transplantation, may not be optimal for pig organs. Ex-vivo lung perfusion (EVLP) systems that keep lungs viable outside the body could be adapted for xenotransplantation, potentially allowing genetic modifications or therapeutic interventions during transport.
Advanced preservation could also address the severe primary graft dysfunction observed in Guangzhou. By maintaining pig lungs in optimal physiological conditions during transport and implantation, it may be possible to reduce the inflammatory response that leads to early graft complications.
Expert Reactions: The Scientific Community Responds
The Guangzhou breakthrough has generated intense discussion within the transplant community, with experts offering both praise and cautionary perspectives on the achievement and its implications for future clinical applications.
European Perspectives Dr. Beatriz Domínguez-Gil, Director General of the Spanish National Transplant Organization and a leading voice in European transplant medicine, characterized the work as “an essential step forward in xenotransplantation research.” However, she emphasized that “refinements in genetic modifications, immunosuppression protocols, and organ preservation techniques will be necessary before considering clinical applications in living patients.”
Her assessment reflects the European regulatory approach, which tends to be more conservative regarding experimental interventions. The European Medicines Agency (EMA) has not yet established clear guidelines for xenotransplantation clinical trials, preferring to wait for more comprehensive preclinical data.
American Expert Analysis Dr. Robert Montgomery, Director of the NYU Langone Transplant Institute and a pioneer in pig kidney xenotransplantation, praised the technical achievement while noting the significant challenges revealed by the experiment. “The occurrence of primary graft dysfunction and antibody-mediated rejection, even with intensive immunosuppression, demonstrates that we still have substantial biological barriers to overcome,” he noted.
Montgomery’s team has conducted several pig kidney transplants in brain-dead patients, providing comparative experience. His assessment suggests that while kidney xenotransplantation may be approaching clinical viability, lung xenotransplantation faces additional hurdles that will require years of additional research.
The consensus from experts at the American Society of Transplantation: this proves pig lung xenotransplantation is possible, but clinical trials in living patients are still years away.
Ethical Considerations: Navigating Uncharted Territory
The success of pig lung xenotransplantation raises profound ethical questions that extend far beyond medical considerations, touching on fundamental issues of human-animal relationships, consent, justice, and resource allocation.
The Consent Paradigm Using brain-dead patients for xenotransplantation research presents unique ethical challenges. While these individuals cannot benefit from the experimental intervention, their participation provides crucial data that could save thousands of future lives. The consent process must balance the experimental nature of the research with the genuine potential for societal benefit.
Families making decisions about experimental xenotransplantation face unprecedented choices. They must understand that their loved one is participating in research that could revolutionize medicine while accepting that there’s no direct benefit to the patient. This creates a form of altruistic consent that differs fundamentally from standard clinical decision-making.
Justice and Access If xenotransplantation becomes clinically viable, questions of access and resource allocation become critical. Will pig organs be available to all patients who need them, or will cost and complexity create new forms of healthcare inequality? The initial costs of xenotransplantation will likely be enormous, potentially creating a two-tiered system where wealthy patients receive unlimited organs while others continue to face scarcity.
International equity poses additional challenges. If pig organs become available in wealthy countries but remain inaccessible in developing nations, xenotransplantation could exacerbate global health disparities rather than resolving the universal organ shortage.
Animal Welfare Considerations Xenotransplantation requires raising genetically modified pigs in sterile, controlled environments for the sole purpose of organ harvest. This raises questions about the moral status of animals bred specifically for human benefit and the conditions under which such breeding is ethically acceptable.
The genetic modifications themselves raise additional questions. By humanizing pig organs, are we creating chimeric animals that exist in a moral gray area between human and animal? What rights, if any, do these genetically modified creatures possess?
Religious and Cultural Perspectives Pig organ transplantation faces significant religious and cultural barriers. Islam and Judaism prohibit pork consumption, and many adherents question whether pig organ transplantation violates religious law. While most Islamic and Jewish authorities have indicated that life-saving medical treatments take precedence over dietary restrictions, acceptance is not universal.
Cultural attitudes toward human-animal boundaries also vary significantly across societies. Some cultures view the incorporation of animal organs into human bodies as fundamentally problematic, while others see it as an acceptable medical intervention.
The Technology Revolution: Convergence of Multiple Breakthroughs
The success in Guangzhou represents the convergence of multiple technological revolutions that have transformed what’s possible in transplant medicine. Each technology contributes essential capabilities that make xenotransplantation feasible.
CRISPR and Genetic Engineering The precision required to make these changes while maintaining organ function demonstrates the sophisticated understanding scientists have developed of cross-species compatibility.
The speed of genetic modification has also revolutionized the field. What once required years of traditional breeding can now be accomplished in months through direct genetic manipulation. This acceleration enables rapid iteration and testing of different genetic configurations.
Advanced Immunosuppression Modern immunosuppressive drugs provide targeted control over specific immune pathways rather than the broad immunosuppression of earlier generations. Biologics like eculizumab and rituximab can block specific immune responses while leaving other protective mechanisms intact.
The combination of multiple targeted therapies creates synergistic effects, allowing more effective immune control with potentially less toxicity than single-agent approaches. Future generations of immunosuppressive agents promise even more precise control over rejection responses.
Ex-Vivo Organ Support EVLP and similar technologies are revolutionizing organ preservation and assessment. These systems can maintain organs outside the body for hours or days, allowing time for therapeutic interventions, genetic modifications, or detailed functional assessment before transplantation.
For xenotransplantation, ex-vivo support could enable real-time monitoring of pig organ function and compatibility before implantation. Organs could be “conditioned” with immunosuppressive agents or anti-inflammatory treatments to reduce rejection risk.
Bioengineering and Regenerative Medicine Tissue engineering approaches could eventually complement or replace xenotransplantation. Scientists are developing techniques to grow human organs from patient cells, potentially eliminating rejection risk entirely. However, these technologies remain years from clinical application and may not provide the immediate solution needed for current patients.
The combination of xenotransplantation with tissue engineering could provide intermediate solutions. Pig organs could serve as biological scaffolds that are gradually replaced with patient cells, creating hybrid organs that combine the immediate availability of xenotransplantation with the compatibility of autologous tissue.
Economic Implications: Transforming Healthcare Markets
Successful xenotransplantation would fundamentally alter healthcare economics, creating new markets while disrupting existing systems. The economic implications extend far beyond the direct costs of organ production and transplantation.
Market Creation and Disruption Xenotransplantation could create entirely new markets worth hundreds of billions of dollars. Companies specializing in genetically modified pig production, specialized surgical techniques, xenotransplantation-specific medications, and long-term monitoring systems would emerge as major healthcare sectors.
Traditional organ procurement organizations (OPOs) would face significant disruption. Their current role in organ allocation and distribution could become less critical if pig organs provide unlimited supply. However, they might evolve to include xenotransplantation coordination and quality control functions.
Cost-Benefit Analysis Initial xenotransplantation procedures will likely cost significantly more than human organ transplants due to the complexity of genetic engineering, specialized surgical techniques, and intensive monitoring requirements. However, economies of scale could eventually reduce costs below current levels.
The economic benefits extend beyond direct transplant costs. End-stage organ disease patients consume enormous healthcare resources while waiting for organs. Dialysis alone costs the US healthcare system over $50 billion annually. Readily available pig kidneys could eliminate most of this expense while providing better outcomes for patients.
Investment and Innovation Venture capital and pharmaceutical companies have invested hundreds of millions of dollars in xenotransplantation research. Companies like eGenesis, Revivicor, and United Therapeutics are racing to develop clinically viable pig organs, with market capitalizations reflecting the enormous commercial potential. The biotechnology approach to producing organs mirrors similar advances in cellular agriculture for food production, where controlled biological systems are revolutionizing traditional industries.
Government investment has also increased dramatically. The National Institutes of Health, Department of Defense, and other agencies recognize xenotransplantation’s potential to address critical medical needs while reducing long-term healthcare costs.
Global Market Dynamics Xenotransplantation could reshape global healthcare dynamics. Countries with advanced biotechnology sectors could become major organ exporters, while nations lacking these capabilities might become dependent on imports. This could create new forms of medical colonialism or, alternatively, democratize access to life-saving organs.
Regulatory harmonization will be crucial for global market development. Different safety standards and approval processes could fragment markets and limit the global impact of xenotransplantation advances.
Timeline to Clinical Reality: Managing Expectations
Based on current research trajectories, expert assessments, and regulatory requirements, xenotransplantation advancement will likely follow a staged progression, with different organs reaching clinical viability at different times. These projections align with broader technology predictions for the 2030s, where biotechnology convergence is expected to transform healthcare delivery fundamentally.
Immediate Future (2025-2027): Foundation Building The next two years will focus on replicating and extending the Guangzhou results. Additional brain-dead patient studies will test refined genetic modifications, improved immunosuppressive protocols, and enhanced preservation techniques. Multiple centers will likely attempt similar procedures, building a database of experience with pig lung xenotransplantation.
Kidney xenotransplantation will likely progress more rapidly, with possible initiation of small-scale clinical trials in living patients. Several patients have already received pig kidneys under compassionate use programs, and formal clinical trials await regulatory approval.
Near-Term Development (2027-2030): Clinical Translation Heart xenotransplantation may reach limited clinical trials during this period, building on previous compassionate use cases and the growing experience with genetic modifications. However, the mechanical complexity of heart function and the availability of artificial heart alternatives may limit early adoption.
Liver xenotransplantation, building on recent auxiliary transplant successes, could also enter clinical testing. The liver’s regenerative capacity and tolerance for partial function make it an attractive target for early clinical applications.
Medium-Term Goals (2030-2035): Lung Applications Lung xenotransplantation will likely require this extended timeline due to the unique challenges identified in Guangzhou. The complexity of respiratory physiology, susceptibility to primary graft dysfunction, and exposure to environmental pathogens create additional hurdles that require comprehensive solutions.
During this period, genetic modifications will likely expand to 10-15 changes per pig, immunosuppressive protocols will become less toxic through targeted therapies, and organ preservation techniques will be optimized for cross-species transplantation.
Long-Term Vision (2035+): Routine Clinical Practice If current research trajectories continue successfully, xenotransplantation could become routine clinical practice within 15 years. This timeline assumes continued scientific progress, regulatory approval, and resolution of safety concerns including virus transmission.
The transformation won’t happen simultaneously across all organs. Kidneys will likely lead adoption, followed by hearts and livers, with lungs potentially remaining experimental for extended periods due to their unique complexity.

The Convergence of Multiple Scientific Revolutions
The Guangzhou breakthrough represents more than just a single medical achievement—it demonstrates the convergence of multiple scientific revolutions that are collectively transforming what’s possible in medicine.
Artificial Intelligence and Machine Learning AI algorithms are increasingly being used to predict organ compatibility, optimize immunosuppressive protocols, and identify genetic modifications that could improve xenotransplantation success. Machine learning models can analyze vast datasets of transplant outcomes to identify patterns and predict which combinations of genetic modifications will be most effective. This convergence of AI and drug discovery is accelerating medical breakthroughs across multiple therapeutic areas.
In the operating room, AI-assisted surgical planning and real-time guidance systems could improve the precision of xenotransplant procedures, reducing complications and improving outcomes. Robotic surgical systems, enhanced with AI capabilities, might eventually perform routine xenotransplant procedures with superhuman precision.
Nanotechnology and Drug Delivery Nanotechnology platforms could revolutionize immunosuppression in xenotransplantation by enabling targeted drug delivery directly to transplanted organs. Nanoparticles could be designed to accumulate specifically in pig organs, delivering immunosuppressive agents locally while minimizing systemic toxicity.
Smart drug delivery systems could also provide real-time monitoring of organ function and automatic adjustment of immunosuppressive therapy based on rejection markers, creating personalized treatment protocols that adapt to individual patient responses.
Quantum Computing and Molecular Modeling As quantum computing becomes more accessible, it could accelerate the discovery of optimal genetic modifications for xenotransplantation. Quantum algorithms could model complex molecular interactions between pig and human proteins, identifying potential compatibility problems before genetic modifications are even attempted.
This computational power could also optimize immunosuppressive drug combinations, predicting which protocols will be most effective for specific genetic modifications and patient characteristics.
Bioprinting and Tissue Engineering While still in early development, bioprinting technologies could eventually complement xenotransplantation by creating hybrid organs that combine pig scaffolds with patient-derived cells. This approach could provide the immediate availability of xenotransplantation with the compatibility benefits of autologous tissue.
Near-term applications might include bioprinted patches for organ repair or enhancement of pig organs with human cellular components to improve integration and function.
Looking Beyond the Headlines: The Nuanced Reality
While the Guangzhou success represents a genuine breakthrough, the reality is more nuanced than popular media coverage suggests. The transplanted lung experienced significant complications that highlight the work still required for clinical application.
The Complications That Matter The severe primary graft dysfunction observed within 24 hours of transplantation represents a major concern. This condition, characterized by fluid accumulation and impaired gas exchange, occurs in human-to-human lung transplants but appears more severe in xenotransplantation. Understanding and preventing PGD will be crucial for future success.
The antibody-mediated rejection episodes on Days Three and Six, while treatable with intensive immunosuppression, demonstrate that current genetic modifications are insufficient to prevent all rejection responses. The recovery from these episodes is encouraging, but the need for such intensive treatment raises questions about long-term viability.
The Immunosuppressive Burden The eight-drug immunosuppressive regimen used in Guangzhou represents a level of immune suppression that would be unacceptable in living patients. The combination of broad-spectrum T-cell depletion, complement inhibition, and multiple small-molecule immunosuppressants creates enormous infection and malignancy risks.
Future protocols must achieve equivalent rejection control with significantly less toxicity. This requires developing more targeted therapies that can selectively control xenogeneic rejection while preserving protective immunity against infections and cancer.
The Preservation Problem The pig lung showed signs of ischemia-reperfusion injury, suggesting that current organ preservation techniques may be suboptimal for xenotransplantation. The longer transport times required for pig organs (due to the need for extensive pathogen testing) exacerbate preservation challenges.
Advanced preservation techniques, including machine perfusion and therapeutic interventions during storage, will likely be necessary for optimal outcomes. These technologies are still in development and may add significant complexity and cost to xenotransplantation procedures.
The Microbiome Factor: An Emerging Complexity
Recent research has revealed that the microbiome—the collection of microorganisms living in and on our bodies—plays a crucial role in transplant outcomes. This discovery adds another layer of complexity to xenotransplantation that researchers are just beginning to understand.
Species-Specific Microbiomes Pigs and humans have fundamentally different microbiomes, adapted to their respective physiologies and environmental exposures. When a pig organ is transplanted into a human, questions arise about how the organ’s associated microorganisms will interact with the human microbiome.
The lung microbiome is particularly complex, as it’s constantly exposed to environmental microorganisms through breathing. A pig lung transplanted into a human will encounter human respiratory tract bacteria, potentially creating novel microbial communities with unpredictable effects on organ function.
Immune System Interactions The microbiome plays a crucial role in training and regulating the immune system. Disruption of normal microbial communities could affect immune responses to transplanted organs, potentially increasing rejection risk or altering the effectiveness of immunosuppressive medications.
Understanding these interactions will be crucial for optimizing xenotransplantation outcomes. Future protocols may need to include microbiome management strategies, using probiotics, targeted antibiotics, or microbial transplantation to optimize the microbial environment around transplanted organs.
The Next Generation: Pediatric Xenotransplantation
While current xenotransplantation research focuses primarily on adult recipients, pediatric applications present unique opportunities and challenges that could shape the future of the field.
Unique Pediatric Advantages Children’s immune systems are more adaptable than adults, potentially making them more tolerant of xenotransplanted organs. The developing immune system might be more capable of accepting pig organs as “self,” reducing long-term rejection risk.
Pediatric patients also have longer potential lifespans, making the durability of xenotransplanted organs more critical but also more impactful. A successful pediatric xenotransplant could provide decades of benefit, making even complex procedures cost-effective over the patient’s lifetime.
Growth and Development Considerations However, pediatric xenotransplantation faces unique challenges related to growth and development. Pig organs must be able to grow with the child or be designed for eventual replacement as the child matures. This might require multiple transplant procedures or organs engineered with growth potential.
The long-term effects of immunosuppression on developing children are also less well understood, requiring careful consideration of protocol design and monitoring strategies.
Environmental and Sustainability Considerations
As xenotransplantation moves toward clinical reality, environmental and sustainability issues are becoming increasingly important considerations that could influence the field’s development and public acceptance.
Carbon Footprint of Organ Production Producing genetically modified pigs for xenotransplantation requires significant resources and energy consumption. The controlled environments necessary for pathogen-free pig breeding consume substantial electricity, while the genetic engineering process requires energy-intensive laboratory procedures.
However, the environmental impact must be weighed against the benefits of eliminating organ shortage. The current healthcare system expends enormous resources maintaining patients with end-stage organ disease through dialysis, mechanical ventilation, and other supportive measures that could be eliminated with adequate organ availability.
Sustainable Production Models Future xenotransplantation programs will need to develop sustainable production models that minimize environmental impact while ensuring organ quality and safety. This might include renewable energy sources for breeding facilities, optimized feed production systems, and waste management strategies that minimize environmental contamination.
The Future Landscape: Multiple Pathways Forward
As xenotransplantation research progresses, multiple technological pathways are emerging that could complement or compete with pig organ transplantation, creating a diverse landscape of options for addressing organ shortage.
Regenerative Medicine Integration The combination of xenotransplantation with regenerative medicine techniques could provide optimal solutions that leverage the advantages of both approaches. Pig organs could serve as biological scaffolds that are gradually replaced with patient-derived cells, providing immediate function while eliminating long-term rejection risk.
Stem cell therapies could be used to enhance pig organ integration, seeding transplanted organs with human cells that improve compatibility and function. This hybrid approach could provide superior outcomes to either technology alone.
Artificial Organ Development Advances in artificial organ technology could provide alternatives to biological xenotransplantation for some applications. Artificial hearts have already achieved limited success, and artificial lung devices are in development that could provide bridging therapy or permanent solutions for some patients.
The choice between artificial and biological organs will likely depend on patient-specific factors, with artificial devices potentially preferred for patients unable to tolerate immunosuppression and biological organs preferred for patients requiring long-term function.
Gene Therapy and Organ Repair Gene therapy approaches that can repair or regenerate damaged organs could reduce the need for organ replacement altogether. Early success in treating genetic forms of organ disease suggests that some conditions currently requiring transplantation might eventually be managed through genetic intervention.
However, gene therapy approaches are likely to be most effective for early-stage disease, with organ replacement remaining necessary for end-stage conditions where irreversible damage has occurred.
Global Impact: Beyond Individual Patient Care
Successful xenotransplantation would extend far beyond individual patient benefits, potentially reshaping healthcare systems, economic structures, and social attitudes toward medical intervention.
Healthcare System Transformation Unlimited organ availability would eliminate transplant waiting lists, fundamentally changing how healthcare systems approach end-stage organ disease. Instead of rationing scarce resources, providers could focus on optimizing outcomes and quality of life.
The economics of organ failure would shift dramatically. Rather than managing patients through prolonged decline while awaiting organs, providers could intervene earlier with definitive treatment. This could improve outcomes while reducing overall healthcare costs.
Regional disparities in organ availability, currently a major factor in transplant outcomes, could be eliminated. Patients would no longer need to relocate to access transplant centers or compete with others for limited organs based on geographic proximity to donors.
Social and Psychological Impact The knowledge that organ failure is no longer a death sentence could fundamentally alter how society approaches chronic disease management. Patients with conditions that progress to organ failure might feel less anxiety about their diagnosis, knowing that treatment options will be available when needed.
Healthcare provider decision-making could also change significantly. The current emphasis on preserving failing organs until the last possible moment might shift toward earlier intervention with organ replacement, potentially providing better outcomes with less suffering.
International Development Implications Xenotransplantation could dramatically alter global health equity. Developing nations, which currently have limited access to organ transplantation due to infrastructure and economic constraints, could potentially leapfrog to advanced transplant capabilities if pig organs become widely available.
However, this transformation would require significant technology transfer, training programs, and international cooperation to ensure that xenotransplantation benefits reach underserved populations rather than exacerbating existing healthcare disparities.
The Road Ahead: From Science Fiction to Medical Reality
The success in Guangzhou represents a watershed moment in transplant medicine—the point where pig-to-human organ transplantation transitioned from theoretical possibility to demonstrated reality. However, the journey from proof-of-concept to routine clinical practice will require sustained effort across multiple scientific disciplines.
Scientific Challenges Requiring Resolution Genetic engineering must advance beyond the current six-modification approach to create pigs with comprehensive human compatibility. This includes not only immune compatibility but also physiological compatibility, addressing differences in organ size, blood flow patterns, and metabolic requirements.
Immunosuppression research must develop protocols that can control xenogeneic rejection while maintaining manageable toxicity profiles. This likely requires moving beyond current broad-spectrum approaches to targeted interventions that specifically address cross-species immune responses.
Infection control strategies must comprehensively address virus transmission risks while maintaining organ viability. This includes not only PERV elimination but also control of other pig pathogens that could potentially affect immunosuppressed recipients.
Regulatory and Ethical Framework Development Regulatory agencies must develop comprehensive frameworks for xenotransplantation oversight that balance innovation with safety. Current guidelines, developed primarily for kidney and heart xenotransplantation, may require modification for lung-specific applications.
International coordination will be essential to ensure safety standards while preventing regulatory arbitrage that could compromise patient safety. The global nature of organ shortage requires coordinated responses that transcend national boundaries.
Ethical frameworks must address the unique challenges of xenotransplantation, including consent processes for experimental interventions, resource allocation strategies for expensive new technologies, and approaches to animal welfare in organ production.
Clinical Implementation Challenges Training programs must prepare surgical teams, intensive care specialists, and transplant coordinators for the unique challenges of xenotransplantation. The complexity of managing cross-species transplants requires specialized expertise that doesn’t currently exist at scale.
Healthcare systems must develop infrastructure for xenotransplantation programs, including specialized surgical facilities, long-term monitoring capabilities, and emergency response protocols for complications unique to pig organ recipients.
Insurance and payment systems must adapt to cover xenotransplantation procedures and their associated long-term monitoring requirements. The cost structures for unlimited organ availability will be fundamentally different from current scarcity-based systems.
Conclusion: A New Chapter in Medical History
The successful pig-to-human lung transplantation performed in Guangzhou represents more than just a medical breakthrough—it marks the beginning of a new chapter in human medicine where the boundaries between species become permeable in service of saving lives.
The nine days that the pig lung functioned in a human chest have fundamentally altered our understanding of what’s possible in transplant medicine. While significant challenges remain, the proof-of-concept has been established: pig organs can sustain human life, and the biological barriers between species, while formidable, are not insurmountable.
The road ahead will be long and complex, requiring continued advances in genetic engineering, immunosuppression, organ preservation, and safety monitoring. Regulatory frameworks must evolve to address the unique challenges of cross-species transplantation, while ethical considerations must be carefully balanced against the potential to save thousands of lives annually.
Perhaps most importantly, the success in Guangzhou has provided something invaluable to the 100,000+ people currently waiting for organ transplants worldwide: hope. Hope that the biological lottery of organ failure need not be a death sentence. Hope that the arbitrary geographical and temporal constraints of human organ availability can be overcome. Hope that future generations might view organ shortage as a historical curiosity rather than a contemporary tragedy.
The convergence of genetic engineering, advanced immunosuppression, artificial intelligence, and regenerative medicine is creating unprecedented opportunities to address one of medicine’s greatest challenges. While xenotransplantation may not be the complete solution to organ shortage, it represents a crucial component of a comprehensive approach that could transform the lives of millions of patients worldwide.
The pig lung that functioned for nine days in a human chest has shown us a glimpse of that future—a future where the question is not whether an organ will be available when needed, but simply which therapeutic approach will provide the best outcome for each individual patient. That future, while still years away, is no longer a matter of if, but when.
For the 17 people who will die today waiting for organ transplants, this timeline may seem frustratingly distant. But for future patients facing organ failure, the work begun in Guangzhou offers the promise of a fundamentally different reality—one where organ failure becomes a treatable condition rather than a terminal diagnosis, and where the boundaries between species dissolve in service of the most fundamental medical mission: saving lives.
The age of xenotransplantation has begun, and with it, a new era of hope for patients facing the ultimate medical challenge. The pig lung that survived nine days in a human chest has shown us that the impossible can become possible, and that the future of transplant medicine may be limited only by our imagination, determination, and commitment to pushing the boundaries of what it means to heal.
Resources and Tools
Stay Updated on Breakthrough Science:
- Beehiiv Newsletter Platform – Build your own science newsletter and stay informed about the latest medical breakthroughs
- vidIQ YouTube Growth – Optimize your science communication and reach more people with life-saving information
Research and Analysis Tools:
- AdCreative.ai – Create compelling visuals to communicate complex medical concepts
- ElevenLabs AI Voice – Generate professional narrations for medical education content
Privacy and Security:
- Surfshark VPN – Secure your medical research and protect sensitive health information online
- NordVPN – Advanced protection for healthcare professionals and researchers
Pingback: Magnetic Micro-Robots Target Kidney Stones - Waterloo 2025