The concept of transforming a patient’s immune system into a personalized cancer-fighting army once belonged to science fiction. Today, CAR-T cell therapy represents one of medicine’s most remarkable achievements: a single treatment that can hunt and eliminate cancer cells for over a decade. This “living drug” approach has fundamentally altered cancer treatment paradigms, offering hope where traditional therapies have failed.
CAR-T therapy extracts a patient’s T-cells, genetically engineers them to recognize specific cancer markers, then reinfuses them as microscopic assassins that patrol the bloodstream indefinitely. Unlike chemotherapy that destroys healthy and cancerous tissue indiscriminately, these engineered cells target only cancer while preserving normal tissue.
The University of Pennsylvania’s pioneering research has documented patients maintaining complete remissions for more than ten years, with modified T-cells still circulating and ready to attack any returning cancer. This durability represents a paradigm shift from managing cancer as a chronic disease to potentially curing it with a single intervention.
The Science Behind Living Drugs
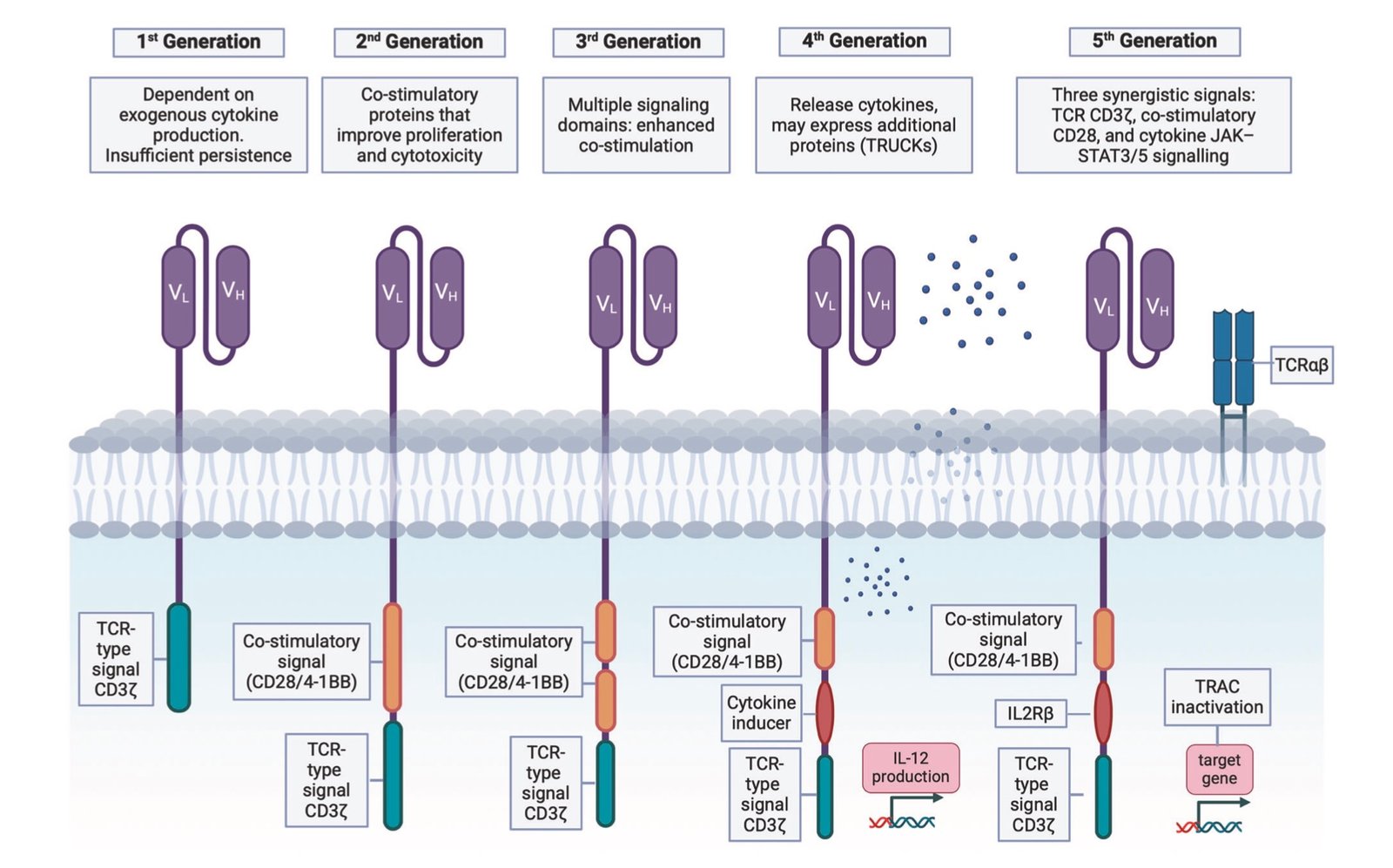
CAR-T therapy begins with leukapheresis, a process that extracts T-cells from the patient’s blood. These cells undergo genetic modification in specialized laboratories, receiving synthetic receptors called Chimeric Antigen Receptors (CARs) that enable recognition of tumor-specific antigens.
The most commonly targeted antigen is CD19, present on B-cell malignancies including acute lymphoblastic leukemia and large B-cell lymphomas. For multiple myeloma, BCMA (B-cell maturation antigen) serves as the primary target. These surface proteins act as molecular signatures that distinguish cancer cells from healthy tissue.
The genetic engineering process involves viral vectors that insert CAR genes into T-cells, programming them with new capabilities. The modified cells are then expanded in culture to achieve therapeutic doses, typically millions to billions of cells per infusion. This manufacturing process, known as “vein-to-vein” time, traditionally required 2-4 weeks but recent advances have reduced this to under two weeks in optimal conditions.
Once reinfused, CAR-T cells encounter their target antigens and activate, releasing cytotoxic compounds that destroy cancer cells. They also proliferate rapidly, expanding their numbers in response to tumor burden. Most remarkably, some CAR-T cells differentiate into memory cells, maintaining long-term surveillance against cancer recurrence.
The mechanism resembles natural immune responses but with enhanced specificity and persistence. Traditional T-cells require complex activation signals and often become exhausted when fighting cancer. CAR-T cells bypass these limitations through engineered co-stimulatory domains that provide sustained activation signals.
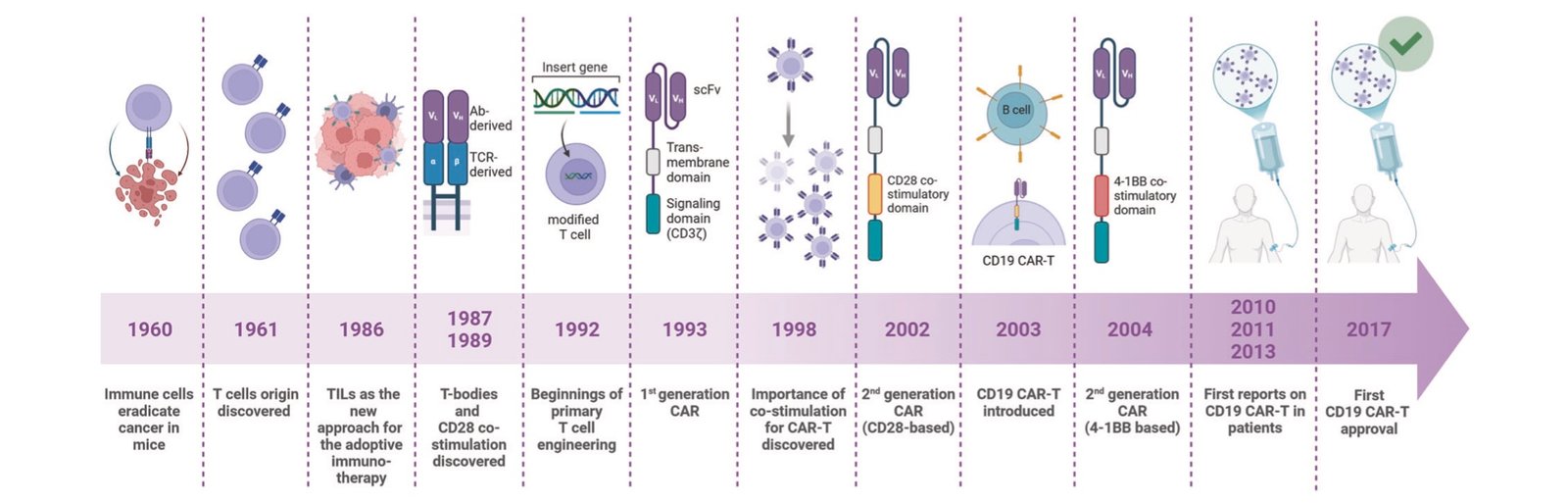
Clinical Outcomes and Long-Term Durability
Clinical evidence for CAR-T efficacy spans multiple cancer types, with the most compelling data emerging from hematologic malignancies. In acute lymphoblastic leukemia, complete remission rates reach 90% in certain patient populations, representing unprecedented success in previously incurable cases.
The ZUMA-1 trial, which established axicabtagene ciloleucel (Yescarta) as standard care for relapsed/refractory large B-cell lymphoma, now reports five-year follow-up data. Approximately 40% of patients remain in complete remission, with many showing no detectable cancer cells. These results establish genuine long-term survivors in a disease that was universally fatal before CAR-T therapy.
Multiple myeloma applications have shown similarly impressive results. Ciltacabtagene autoleucel (Carvykti) demonstrates that roughly one-third of patients remain alive and progression-free five years after treatment. The FDA’s 2024 approval for earlier treatment lines reflects growing confidence in the therapy’s benefit-risk profile.
Real-world evidence studies consistently validate clinical trial results, though with slightly lower response rates reflecting more heterogeneous patient populations. Elderly patients and those with significant comorbidities show reduced efficacy compared to carefully selected trial participants, but substantial numbers still achieve durable remissions.
Treatment failures typically result from three mechanisms: antigen escape (cancer cells lose the target protein), T-cell exhaustion (engineered cells become dysfunctional), or immunosuppressive tumor microenvironments that inactivate CAR-T cells. Understanding these resistance patterns has informed next-generation CAR designs and combination treatment strategies.
The durability question extends beyond mere survival statistics. Quality of life analyses show that CAR-T responders often return to normal activities without ongoing treatment requirements, contrasting sharply with traditional approaches requiring continuous therapy. This functional cure concept represents a fundamental shift in cancer care philosophy.
Regulatory Evolution and Access Improvements
The regulatory landscape for CAR-T therapy has evolved rapidly, reflecting both growing experience with safety management and recognition of the treatment’s transformative potential. The FDA’s June 2025 elimination of Risk Evaluation and Mitigation Strategies (REMS) requirements marks a watershed moment for patient access.
REMS programs previously required extensive administrative procedures, including mandatory training for healthcare providers and restricted distribution systems. These requirements, while important for early safety monitoring, created significant barriers for treatment centers and delayed patient access. The elimination reflects confidence gained from treating over 30,000 patients worldwide.
However, safety vigilance remains paramount. The FDA’s April 2024 boxed warning for secondary T-cell malignancies represents the most significant safety concern identified through post-market surveillance. European Medicine Agency data quantifies this risk at approximately 38 cases among 42,500 treated patients, representing a very low but non-zero incidence rate.
These secondary malignancies appear to result from the genetic modification process itself, where viral vectors occasionally insert CAR genes into oncologically sensitive genomic regions. While the absolute risk remains minimal compared to cancer treatment benefits, long-term monitoring protocols now extend to 15 years post-treatment.
The regulatory streamlining has practical implications for patient care. Treatment center certification requirements have been simplified, potentially expanding the network of qualified facilities. The previous requirement for patients to remain near treatment centers for four weeks has been reduced to two weeks, significantly reducing logistical burdens.
International regulatory harmonization efforts are progressing, with the European Medicines Agency and Japan’s PMDA adopting similar but not identical approval pathways. These differences create complexities for global development programs but reflect varying healthcare system priorities and risk tolerance levels.
Manufacturing Revolution and Technological Advances
The transformation of CAR-T manufacturing represents one of biotechnology’s most impressive industrial achievements. Early production methods required weeks of manual cell culture in centralized facilities, creating bottlenecks that limited patient access and risked product failure due to disease progression during manufacturing delays.
Modern automated manufacturing platforms have revolutionized this process. Companies like Novartis have demonstrated “fast CAR” production achieving complete manufacturing in as little as 24 hours, though end-to-end delivery still requires quality testing and logistics coordination. Point-of-care manufacturing systems enable local production at treatment centers, potentially reducing vein-to-vein times to under one week.
These manufacturing advances carry clinical significance beyond convenience. Studies demonstrate that longer manufacturing times correlate with worse patient outcomes, likely reflecting disease progression during treatment delays. Patients whose disease advances while waiting for CAR-T cells often become ineligible for treatment or experience reduced efficacy.
Non-viral gene editing approaches represent the next technological frontier. CRISPR-based systems can potentially eliminate viral vector safety concerns while enabling more precise genetic modifications. Base editing and prime editing techniques allow targeted genetic changes without creating double-strand DNA breaks, potentially reducing unintended consequences.
Allogeneic or “off-the-shelf” CAR-T products aim to eliminate patient-specific manufacturing entirely. These products use T-cells from healthy donors, genetically modified to reduce graft-versus-host disease risk while maintaining anti-cancer activity. Early clinical trials show encouraging response rates, though long-term persistence remains questionable compared to autologous products.
The most ambitious technological development involves in-vivo CAR-T generation using lipid nanoparticles or viral vectors to deliver genetic modifications directly to patients’ T-cells. This approach could theoretically reduce CAR-T therapy to a simple injection, eliminating manufacturing complexities entirely. First-in-human trials began in 2024, but clinical efficacy data remains limited.
Expanding Applications Beyond Hematologic Cancers
While CAR-T therapy has achieved remarkable success in blood cancers, solid tumor applications remain challenging. The tumor microenvironment in solid cancers creates immunosuppressive conditions that inactivate CAR-T cells. Additionally, solid tumors often lack uniform antigen expression, enabling cancer cells to escape by losing target proteins.
Despite these challenges, encouraging results have emerged in specific contexts. Glioblastoma trials using CAR-T cells targeting multiple antigens simultaneously have shown tumor shrinkage in approximately two-thirds of patients. While durability remains limited compared to hematologic applications, these results demonstrate proof-of-principle for solid tumor CAR-T therapy.
The expansion into autoimmune diseases represents perhaps the most intriguing CAR-T application beyond cancer. Systemic lupus erythematosus patients treated with CD19-targeted CAR-T therapy have achieved drug-free remissions lasting over a year. The mechanistic rationale involves eliminating autoreactive B-cells that drive autoimmune pathology, essentially “resetting” the immune system.
Similar to advances in precision gene editing technologies, these autoimmune applications demonstrate how targeted cellular interventions can address complex biological processes. Early results in myasthenia gravis and multiple sclerosis show substantial clinical improvement, with patients discontinuing immunosuppressive medications while maintaining disease remission.
The success in autoimmune diseases has attracted significant investment and clinical development activity. Multiple pharmaceutical companies have initiated programs targeting various autoimmune conditions, recognizing the potential to transform treatment paradigms for chronic diseases affecting millions of patients globally.
Combination therapy strategies aim to overcome solid tumor challenges through complementary approaches. CAR-T cells combined with checkpoint inhibitors, bispecific antibodies, or tumor microenvironment-modifying agents show promise in early trials. These rational combinations address different aspects of cancer immune evasion simultaneously.
Economic Realities and Healthcare System Integration
The economic implications of CAR-T therapy extend far beyond the headline-grabbing list prices of $400,000 to $500,000 per treatment. Comprehensive economic analyses must consider the total cost of care over patient lifetimes, including avoided expenses from traditional treatments and improved quality-adjusted life years.
Cost-effectiveness studies consistently demonstrate favorable outcomes for CAR-T therapy in appropriate patient populations. The Institute for Clinical and Economic Review (ICER) found that lisocabtagene maraleucel achieves approximately $68,000 per quality-adjusted life-year from a societal perspective, well within conventional cost-effectiveness thresholds.
These analyses become more compelling when considering the alternative costs of managing relapsed/refractory cancers. Traditional salvage therapies often require multiple treatment cycles, prolonged hospitalizations, and supportive care for treatment-related toxicities. The cumulative costs can exceed CAR-T therapy expenses while providing inferior outcomes.
Manufacturing costs represent a fraction of list prices, though exact figures remain proprietary. Industry analyses suggest actual production costs range from $20,000 to $50,000 per dose, with the remainder reflecting research and development investments, facility amortization, and profit margins. The pricing model resembles other curative therapies where value derives from long-term benefit rather than production complexity.
Healthcare system capacity constraints present ongoing challenges despite manufacturing improvements. Treatment requires specialized facilities with intensive care capabilities, experienced nursing staff, and 24/7 physician coverage for managing acute toxicities. Geographic access remains limited, with rural and underserved populations facing significant barriers.
Insurance coverage patterns have generally favored CAR-T therapy for approved indications, though prior authorization requirements often delay treatment initiation. The elimination of REMS requirements should streamline coverage decisions by reducing administrative complexity for payers and providers.
Real-world outcome registries provide valuable data for refining economic models and identifying factors associated with treatment success. These databases reveal concerning disparities in access based on race, geographic location, and socioeconomic status, highlighting ongoing equity challenges in precision medicine delivery.
Safety Profile Evolution and Management Strategies
The safety profile of CAR-T therapy has evolved considerably since initial approvals, with improved understanding enabling more sophisticated risk management strategies. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) remain the primary acute toxicities, but standardized management protocols have significantly improved outcomes.
CRS results from massive cytokine release following CAR-T cell activation and tumor destruction. Symptoms range from fever and hypotension to multi-organ failure requiring intensive care support. The severity correlates with tumor burden and CAR-T cell expansion, making risk stratification possible through pre-treatment assessments.
Tocilizumab, an IL-6 receptor antagonist, has revolutionized CRS management by blocking the primary inflammatory mediator without impairing CAR-T cell function. Early intervention with tocilizumab and corticosteroids has reduced severe CRS incidence and duration, enabling outpatient CAR-T administration at experienced centers.
ICANS presents as confusion, seizures, and other neurological symptoms occurring days to weeks after infusion. The pathophysiology involves blood-brain barrier disruption and neuroinflammation, though precise mechanisms remain unclear. Management relies primarily on corticosteroids and supportive care, with most cases resolving completely.
The secondary T-cell malignancy concern represents a more complex long-term safety consideration. These rare cancers appear to result from CAR gene integration near oncogenes or tumor suppressor genes, though causality remains incompletely established. The extremely low incidence rate must be weighed against the mortality risk of untreated cancer.
Manufacturing safety has improved through enhanced quality control measures and standardized production protocols. Product sterility testing, genetic stability assessments, and cellular viability measurements ensure consistent product quality. Automated manufacturing systems reduce human error risk while maintaining reproducible production standards.
Patient selection criteria have refined based on accumulated experience, identifying factors associated with increased toxicity risk. Patients with high tumor burden, organ dysfunction, or active infections require careful evaluation and potentially disease burden reduction before CAR-T therapy. These refinements have improved safety profiles while maintaining efficacy.
Future Technological Horizons
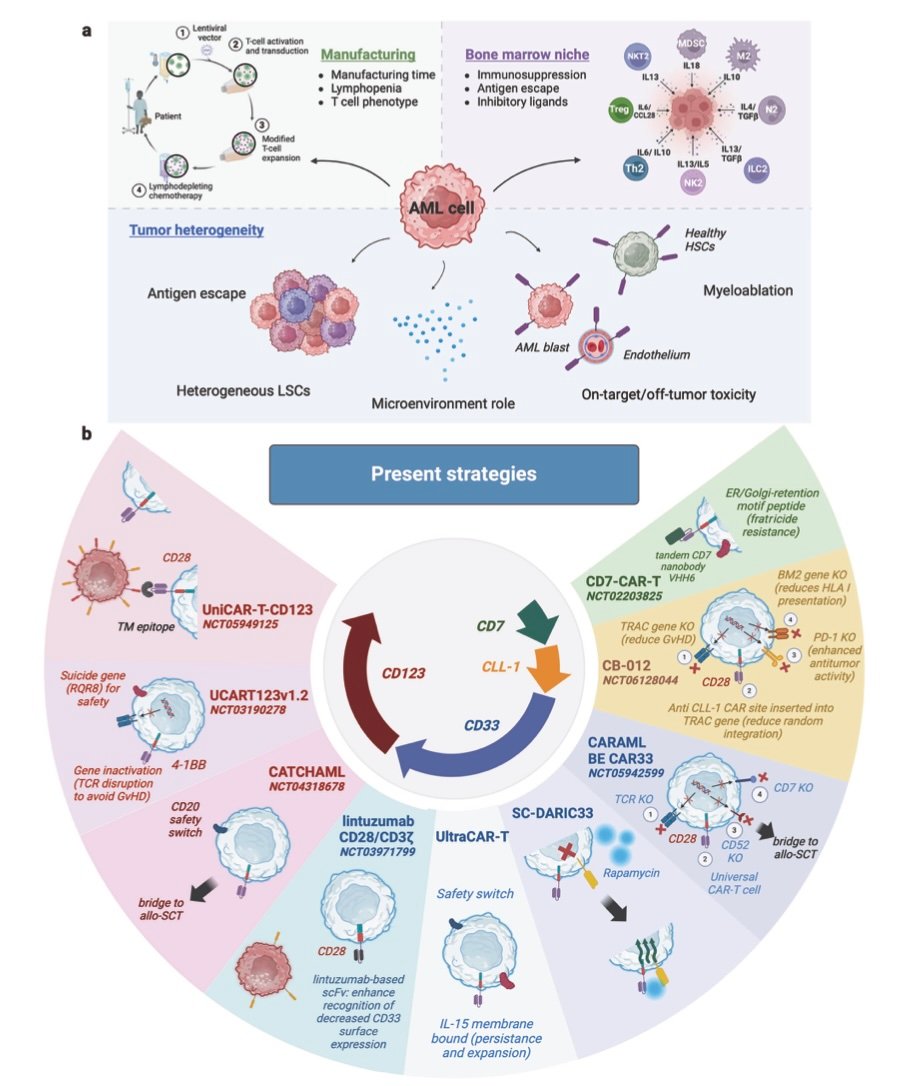
The technological trajectory of CAR-T therapy points toward increasingly sophisticated and accessible treatments. Next-generation CAR designs incorporate multiple safety switches, enhanced trafficking capabilities, and resistance to tumor microenvironment suppression. These improvements aim to expand efficacy while reducing toxicity risks.
Universal CAR-T platforms using allogeneic donors could democratize access by eliminating patient-specific manufacturing. Current approaches use gene editing to remove major histocompatibility antigens while preserving anti-tumor function. Early clinical results show promise, though persistence duration remains shorter than autologous products.
In-vivo CAR-T generation represents the ultimate accessibility goal, potentially reducing the entire treatment to a simple injection. Lipid nanoparticle systems similar to mRNA vaccines could deliver CAR genes directly to patients’ T-cells, programming them within the body. While conceptually elegant, clinical proof-of-concept data remains limited.
Artificial intelligence integration promises to optimize multiple aspects of CAR-T therapy, from patient selection to manufacturing optimization. Machine learning algorithms could predict treatment responses, identify optimal CAR designs, and guide personalized dosing strategies. The convergence of AI-driven drug discovery with cellular therapeutics represents a powerful synergy for advancing precision medicine.
Base editing and prime editing technologies enable more precise genetic modifications than traditional gene therapy approaches. These methods could reduce off-target effects while enabling complex genetic circuits that enhance CAR-T cell function. The safety improvements could be particularly valuable for pediatric applications and autoimmune diseases.
Combination strategies with other immunotherapies continue expanding, recognizing that single-agent approaches may have inherent limitations. CAR-T cells combined with checkpoint inhibitors, cancer vaccines, or oncolytic viruses could address multiple escape mechanisms simultaneously, potentially improving outcomes in challenging applications like solid tumors.
Similar to innovations in medical robotics for targeted interventions, CAR-T therapy represents the convergence of engineering and biology to create precise therapeutic interventions. The parallel development of bioengineered organ transplants demonstrates how cellular engineering approaches are transforming multiple medical fields simultaneously.
Critical Assessment and Remaining Challenges
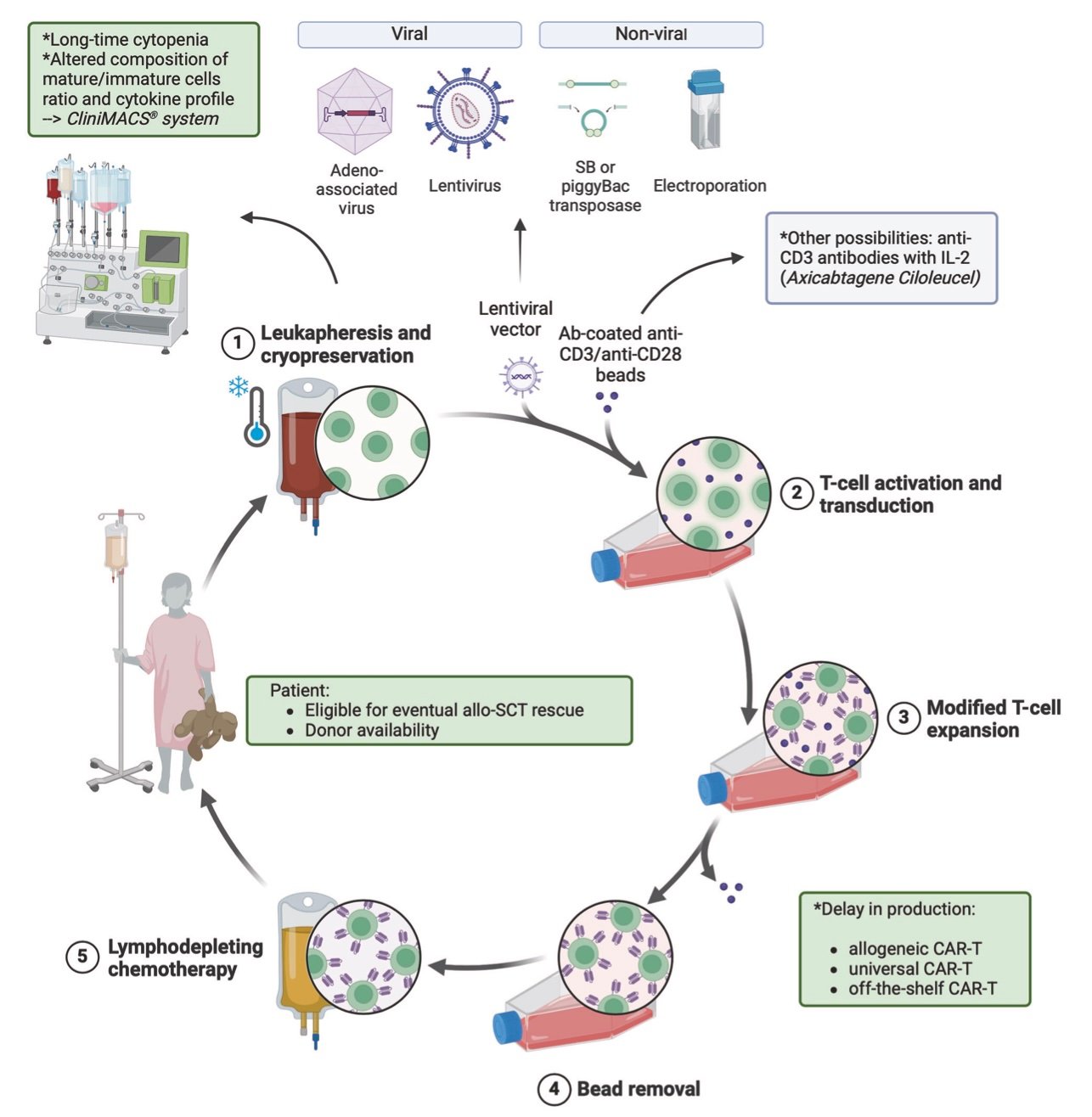
Despite remarkable clinical successes, CAR-T therapy faces significant limitations that require honest acknowledgment. The technology remains most effective in hematologic malignancies, with solid tumor applications showing modest success despite extensive research investment. The tumor microenvironment challenges that limit solid tumor efficacy remain incompletely solved.
Manufacturing complexity continues constraining access despite technological advances. The requirement for specialized facilities and expertise limits global scalability, particularly in resource-constrained healthcare systems. The high costs associated with complex manufacturing and specialized delivery create equity concerns that extend beyond individual patient access.
Resistance mechanisms pose ongoing clinical challenges, with many patients eventually experiencing disease recurrence. Antigen escape, T-cell exhaustion, and immune suppression represent fundamental biological barriers that next-generation approaches aim to address. The development of resistance patterns requires careful monitoring and salvage treatment strategies.
Long-term safety monitoring remains incomplete given the relatively recent introduction of these therapies. While current safety profiles appear favorable, the full spectrum of late effects may not emerge for decades. The secondary malignancy concern, while rare, underscores the importance of extended surveillance programs.
The expansion into autoimmune diseases, while promising, requires careful evaluation of risk-benefit ratios in non-fatal conditions. The immune suppression required for successful treatment could potentially increase infection risks or autoimmune complications. Long-term follow-up studies are essential for establishing safety profiles in these applications.
Quality of life assessments, while generally positive, require more comprehensive evaluation across diverse patient populations. The acute toxicity period can be severe, and some patients experience prolonged recovery phases that impact daily functioning. Patient-reported outcome measures should inform treatment selection and counseling processes.
The Path Forward
CAR-T therapy represents a watershed moment in cancer treatment, transforming the theoretical concept of harnessing the immune system into clinical reality. The ability to create personalized cancer treatments from patients’ own cells exemplifies precision medicine at its most sophisticated level.
The regulatory evolution from restricted access to streamlined delivery reflects growing confidence in safety management and clinical benefit. The elimination of REMS requirements should expand treatment center networks and reduce barriers for appropriate patients. Continued regulatory refinement will likely focus on optimizing benefit-risk ratios while maintaining safety vigilance.
Manufacturing advances continue progressing toward more accessible and efficient production methods. The goal of transforming complex cellular engineering into routine medical procedures requires sustained innovation in automation, quality control, and distribution systems. Point-of-care manufacturing and in-vivo generation approaches could eventually democratize access to these transformative therapies.
The expansion beyond cancer into autoimmune diseases opens entirely new therapeutic frontiers. The mechanistic success in resetting dysfunctional immune systems suggests broader applications in inflammatory and autoimmune conditions. These developments could transform treatment paradigms for millions of patients with chronic autoimmune diseases.
The integration of artificial intelligence, advanced gene editing, and cellular engineering promises even more sophisticated future treatments. The convergence of these technologies could enable treatments tailored to individual patient genetics, tumor characteristics, and immune system profiles. The potential for truly personalized medicine becomes increasingly realistic.
The economic value proposition, while challenging due to high upfront costs, appears favorable when considering long-term outcomes. The potential for functional cures in previously incurable diseases justifies significant healthcare investment. As manufacturing costs decline and access expands, cost-effectiveness ratios should improve substantially.
CAR-T therapy exemplifies how fundamental scientific discoveries can transform medical practice within remarkably short timeframes. From initial proof-of-concept studies to routine clinical use occurred within approximately two decades, demonstrating the acceleration possible when breakthrough science meets clinical need.
The success of CAR-T therapy validates investment in cellular and gene therapy approaches more broadly. The platforms and technologies developed for CAR-T applications are being adapted for other diseases, creating a foundation for next-generation cellular therapeutics. The ripple effects extend far beyond oncology into multiple medical specialties.
For patients facing previously incurable cancers, CAR-T therapy offers genuine hope for long-term survival and functional cure. The transformation from fatal diagnosis to durable remission represents one of medicine’s most remarkable achievements. As access expands and technology advances, these benefits should reach increasingly broad patient populations.
The living drug concept fundamentally challenges traditional pharmaceutical models based on chronic treatment approaches. The ability to engineer therapeutic cells that persist and adapt within patients represents a new paradigm for medicine. This approach may prove applicable to many diseases beyond cancer, potentially revolutionizing how we approach chronic and degenerative conditions.
Essential Tools for Staying Informed on Medical Breakthroughs
To stay current with rapidly evolving medical technologies like CAR-T therapy, researchers and healthcare professionals rely on specialized tools for content creation, research, and communication:
📹 Video Content Creation:
- Fliki – Turn Text into Video (20% OFF) – Transform complex medical research into accessible educational videos
- Pictory – AI Video Creation (20% OFF with Code: CuriosityAI) – Create professional medical content for patient education and professional development
🎙️ Audio and Communication:
- ElevenLabs – Realistic AI Voiceovers – Generate professional narration for medical educational content
- Beehiiv – Newsletter Platform – Share breakthrough medical discoveries with colleagues and patients
📊 Research and Analytics:
- vidIQ – Content Optimization – Track trending medical topics and optimize educational content reach
- Surfshark VPN – Secure Research Access – Access international medical databases and research publications securely
These tools enable medical professionals to effectively communicate complex breakthrough technologies while maintaining scientific accuracy and professional standards.
