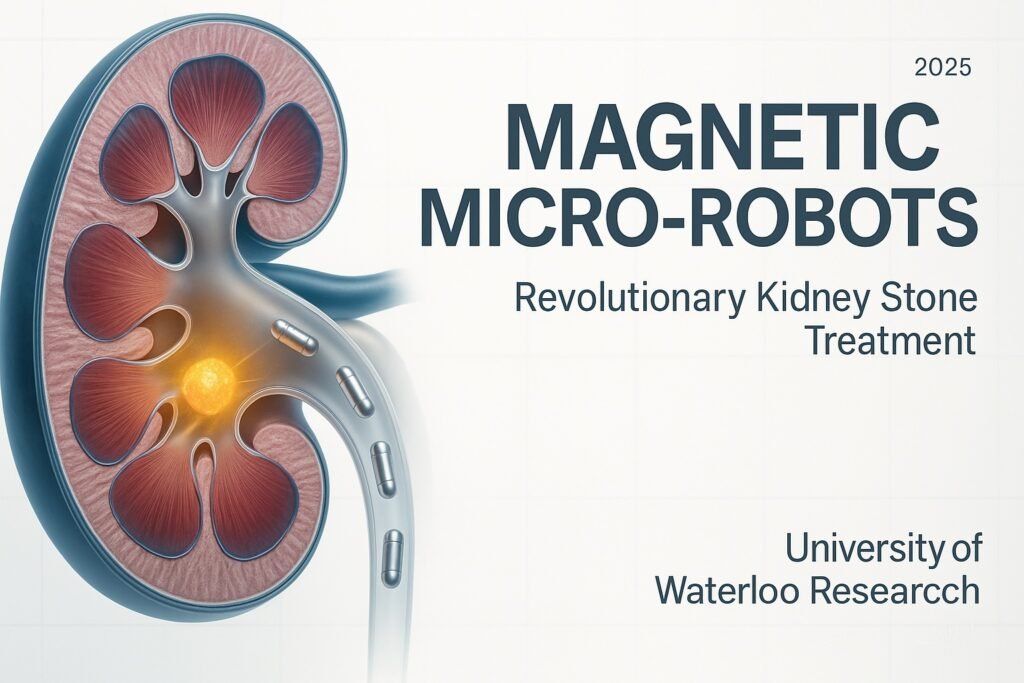
Scientists at the University of Waterloo have developed tiny magnetic robots that swim through the human urinary tract to dissolve kidney stones from the inside. Published in Advanced Healthcare Materials in July 2025, this breakthrough represents a potential paradigm shift from invasive surgical procedures to targeted enzymatic treatment.
The technology addresses a significant medical challenge: over 12% of the global population suffers from kidney stones, with high recurrence rates and limited non-surgical treatment options for certain stone types. Current treatments like extracorporeal shock wave lithotripsy (ESWL) show variable success rates of 30-90% depending on stone characteristics, while surgical interventions carry inherent risks and recovery times.

The Technology Behind Microscopic Medical Navigation
The magnetic micro-robots are engineered as flexible, spaghetti-like strips measuring approximately 1 x 1 x 12 millimeters. Each robot consists of a gelatin-based hydrogel polymer embedded with micromagnets ranging from 0.5 to 0.7 millimeters in diameter. The critical innovation lies in encapsulating urease enzyme within the robot structure, creating a targeted therapeutic delivery system.
Dr. Veronika Magdanz, leading the research team, explains the navigation system utilizes a motorized external magnet mounted on a robotic arm, combined with real-time clinical ultrasound imaging for precise positioning. This dual-control mechanism enables clinicians to guide the tetherless robots through the complex anatomy of the urinary tract with millimeter-scale accuracy.
The urease enzyme serves as the therapeutic payload, catalyzing the hydrolysis of urea naturally present in human urine. This biochemical reaction produces ammonia and carbon dioxide, increasing local pH from typical stone-forming levels around 6.0 to more alkaline conditions of 7.0-7.2. This pH elevation specifically targets uric acid kidney stones, causing them to dissolve naturally rather than requiring mechanical fragmentation.

Laboratory Validation and Clinical Promise
Initial testing conducted in 3D-printed, life-size urinary tract models filled with synthetic human urine demonstrated compelling results. The robots successfully navigated to target locations and maintained therapeutic enzyme activity. Most significantly, kidney stone analogs lost an average of 30% of their weight over five days, sufficient for natural passage through the urinary system.
The pH changes induced by the urease-loaded robots remained stable for up to three months in laboratory conditions, suggesting sustained therapeutic effects. This duration far exceeds traditional oral alkalinization therapies, which often struggle with patient compliance and systemic side effects.
However, these laboratory results represent only the initial proof-of-concept phase. The controlled environment of synthetic urine and static models cannot fully replicate the dynamic conditions within living human anatomy, including fluid flow, peristaltic contractions, and individual anatomical variations.
Competitive Landscape and Alternative Approaches
The University of Waterloo technology enters a crowded field of kidney stone innovations. Similar to breakthrough medical robotics developments like Stanford’s CRISPR-GPT gene editing systems, this represents the convergence of multiple advanced technologies for targeted therapeutic applications.
Alternative non-invasive approaches include the University of Washington’s Burst Wave Lithotripsy (BWL) and Ultrasonic Propulsion systems, which already have randomized clinical trial data showing effectiveness in stone fragmentation and movement. These competing technologies target broader stone types and have advanced further in clinical validation.
The recent pig-to-human lung transplant breakthrough demonstrates how biomedical engineering innovations require extensive pre-clinical validation before human application. Similarly, the Waterloo micro-robots face significant translation challenges from laboratory models to clinical reality.
Other research groups at ETH Zurich, Max Planck Institute, and Columbia University are developing magnetically controlled micro-robots for various medical applications, though none have specifically targeted kidney stone dissolution. Caltech researchers have successfully demonstrated acoustic micro-robots for bladder tumor treatment in living models, proving the general feasibility of microrobotic interventions in urological applications.
Technical Challenges and Safety Considerations
The transition from laboratory success to clinical application faces several critical hurdles. Biocompatibility remains the primary concern, as the long-term interaction between hydrogel materials, embedded magnets, and urinary tract tissues requires thorough evaluation in living systems.
Magnetic field safety presents another consideration, particularly for patients with existing ferromagnetic implants such as surgical clips or stents. While the research team has not disclosed specific magnetic field strengths, similar microrobotic systems operate safely at field levels around 5 millitesla, well below established medical exposure limits according to the International Commission on Non-Ionizing Radiation Protection.
Navigation precision represents perhaps the greatest technical challenge. Guiding millimeter-scale robots through the tortuous anatomy of ureters and kidney calyces while contending with fluid dynamics and peristaltic motion requires sophisticated control algorithms and real-time feedback systems. The team acknowledges ongoing work to refine these control mechanisms before advancing to animal studies.
Manufacturing scalability poses additional challenges. Current laboratory methods using 3D-printed molds for gelatin filament production cannot support commercial-scale requirements. Industrial manufacturing processes must maintain precise dimensions, magnet placement, and enzyme concentration while achieving cost-effective production volumes.

Market Limitations and Clinical Applications
The technology specifically targets uric acid kidney stones, which constitute approximately 5-10% of all kidney stones in most populations. This represents a significant limitation compared to treatments addressing calcium oxalate stones, the most common variety comprising 70-80% of cases.
According to the National Institute of Diabetes and Digestive and Kidney Diseases, uric acid stones are more prevalent in certain populations and conditions, including patients with gout, diabetes, or chronic dehydration. These patients often struggle with traditional treatments, creating a defined clinical niche for targeted enzymatic dissolution.
The global kidney stone management market, valued at approximately $2.55 billion in 2024 and projected to reach $4.02 billion by 2034, demonstrates substantial commercial potential despite the technology’s focused application scope.
Regulatory Pathway and Timeline Reality
The research team has announced large animal studies as their next development phase, with no published results from living biological systems yet available. This represents a critical milestone that will determine the technology’s viability and provide essential safety data for regulatory submissions.
Following successful animal validation, the pathway to human clinical trials would likely require Investigational Device Exemption (IDE) approval from the FDA or Investigational Testing Authorization (ITA) from Health Canada. The regulatory classification would probably involve either a 510(k) submission claiming substantial equivalence to existing magnetic navigation systems or a De Novo classification for novel low-to-moderate risk devices.
Realistic commercialization timelines for novel medical devices typically span 5-10 years from successful pre-clinical data to market approval, similar to timelines seen in AI drug discovery breakthroughs and advanced medical device developments.
Research Team and International Collaboration
The multidisciplinary research team combines expertise from the University of Waterloo’s departments of Systems Design Engineering, Electrical and Computer Engineering, and Mechanical and Mechatronics Engineering. International collaborators from universities and hospitals in Spain and Germany provide clinical perspective and validation of medical relevance.
This collaborative approach mirrors successful biomedical innovations that require integration of engineering expertise with clinical knowledge. The involvement of practicing urologists ensures the technology addresses real medical needs rather than theoretical possibilities.
The research has been published in Advanced Healthcare Materials, a peer-reviewed journal with rigorous scientific standards. This validation by the broader academic community confirms the quality and reproducibility of the underlying research.
Biocompatibility Deep Dive: Materials Science Considerations
The selection of gelatin-based hydrogel polymers for the robot construction represents a careful balance between functionality and biocompatibility. Gelatin, derived from collagen, demonstrates excellent biocompatibility in medical applications, with established use in drug delivery systems and tissue engineering scaffolds.
However, the embedded micromagnets introduce complexity not typically encountered in conventional hydrogel applications. The magnetic materials must maintain their properties while avoiding corrosion or particle shedding within the urinary tract environment. The choice of magnetic material composition—likely neodymium or ferrite-based compounds—requires coating or encapsulation to prevent direct tissue contact.
Long-term retention studies will be critical for regulatory approval. While the robots are designed for natural passage, scenarios involving incomplete elimination or fragmentation must be thoroughly evaluated. The urinary tract’s self-cleaning mechanisms through urine flow may facilitate removal, but retention in kidney calyces or ureter strictures could pose complications.
The enzyme encapsulation technology presents additional biocompatibility considerations. Urease stability within the hydrogel matrix affects both therapeutic efficacy and potential immunogenicity. The protein’s exposure during robot dissolution could trigger immune responses in sensitive patients, requiring careful monitoring during clinical trials.
Economic Analysis and Healthcare System Integration
The economic implications of magnetic micro-robot therapy extend beyond device costs to encompass entire treatment paradigms. Traditional kidney stone management involves multiple interventions, emergency department visits, and extended recovery periods, creating substantial healthcare system burdens.
Current treatment costs vary significantly by methodology and geography. Extracorporeal shock wave lithotripsy procedures range from $6,605 to $11,754 in various US regions, often requiring multiple sessions for complete stone clearance. Ureteroscopic interventions typically cost more due to operating room requirements and specialized equipment, while percutaneous nephrolithotomy involves even higher expenses due to surgical complexity and hospitalization needs.
The micro-robot approach could potentially reduce overall treatment costs by eliminating repeat procedures and minimizing recovery time. However, initial implementation costs include specialized magnetic navigation equipment, ultrasound imaging systems, and physician training programs. Healthcare facilities would need capital investments in robotic control systems and procedure room modifications.
Insurance reimbursement presents a significant market access challenge. Novel medical devices typically enter the market under Category III CPT codes, which provide limited reimbursement while evidence generation occurs. The transition to routine coverage requires demonstration of clinical superiority and cost-effectiveness through randomized controlled trials and real-world evidence studies.
The technology’s focus on uric acid stones, while limiting market size, may actually facilitate insurance approval by targeting a patient population with demonstrated treatment challenges. Patients with recurrent uric acid stones often require multiple interventions and chronic medical management, creating clear economic incentives for effective single-treatment solutions.
Patient Experience and Quality of Life Implications
The patient experience represents a crucial differentiator for micro-robot therapy compared to existing treatments. Traditional kidney stone interventions often involve significant pain, both during the procedure and recovery period. Shock wave lithotripsy, while non-invasive, frequently causes discomfort during treatment and subsequent pain as fragments pass through the urinary tract.
Ureteroscopic procedures, though more precise, require general anesthesia and involve direct instrumentation of the urinary tract. Patients may experience post-operative pain, bleeding, and temporary urinary symptoms. Recovery periods typically span several days to weeks, with activity restrictions and potential complications.
The micro-robot approach promises a fundamentally different patient experience. The minimally invasive catheter-based delivery system could potentially be performed under local anesthesia or conscious sedation. The targeted enzymatic dissolution mechanism aims to reduce stone size gradually, potentially minimizing the traumatic passage of fragments.
However, patient acceptance of robotic medical interventions varies considerably. Some patients may experience anxiety about having autonomous devices within their bodies, even temporarily. Clear communication about the robot’s biocompatible materials, natural elimination pathway, and safety monitoring becomes essential for treatment acceptance.
The psychological benefit of avoiding surgery represents a significant quality-of-life consideration. Many kidney stone patients develop anxiety about recurrent episodes and invasive treatments. A minimally invasive option that addresses the problem at its source could provide substantial psychological relief and improved long-term outlook.
Manufacturing and Scale-Up Challenges
The transition from laboratory prototypes to commercial-scale manufacturing presents complex engineering and quality control challenges. Current gelatin filament production using 3D-printed molds suits research applications but cannot support the precision and volume requirements of medical device manufacturing.
Industrial-scale production must maintain tight tolerances for robot dimensions, magnet placement, and enzyme loading. Variability in any of these parameters could affect navigation accuracy, therapeutic efficacy, or safety profiles. Quality control systems must verify magnetic field strength, enzyme activity, and structural integrity for each device.
Sterilization processes present unique challenges for the combination of organic hydrogel materials and embedded magnets. Traditional gamma irradiation or ethylene oxide sterilization methods may affect enzyme activity or hydrogel properties. Alternative sterilization approaches, such as electron beam or plasma sterilization, require validation for this specific device combination.
Supply chain considerations include sourcing pharmaceutical-grade urease enzyme and maintaining cold chain storage throughout distribution. The enzyme’s temperature sensitivity may require specialized packaging and shipping protocols, adding complexity and cost to the manufacturing process.
Regulatory compliance for manufacturing operations requires Good Manufacturing Practice (GMP) certification and regular inspection by health authorities. The combination of biological materials (enzyme) and medical device components may require dual regulatory oversight, complicating the manufacturing approval process.
Critical Assessment and Future Prospects
The University of Waterloo’s micro-robot platform represents genuine innovation in targeted kidney stone treatment, yet several factors temper immediate clinical expectations. The technology remains in early pre-clinical development, with no animal testing results yet published.
The narrow therapeutic scope limiting application to uric acid stones significantly constrains market potential compared to broader-spectrum treatments. Manufacturing scalability, long-term biocompatibility, and navigation precision in living systems remain unproven challenges requiring extensive validation.
However, the technology addresses a legitimate medical need for patients who respond poorly to conventional treatments. The minimally invasive approach delivered via standard catheter insertion offers clear advantages over surgical alternatives for appropriate candidates.
The research team’s measured approach, emphasizing thorough pre-clinical validation before advancing to human studies, demonstrates appropriate scientific rigor. This contrasts with overly optimistic timelines sometimes associated with early-stage medical technologies.
Investment considerations must weigh the technology’s innovation potential against substantial development risks and lengthy commercialization timelines. The absence of published intellectual property filings creates uncertainty about competitive positioning and commercial defensibility.
Conclusion
The University of Waterloo’s magnetic micro-robots represent a thoughtful engineering approach to a persistent medical challenge. While significant hurdles remain before clinical application, the technology’s targeted mechanism and minimally invasive delivery method address genuine limitations of current kidney stone treatments.
Success will depend on demonstrating safety and efficacy in animal models, followed by careful progression through human clinical trials and regulatory approval processes. The 5-10 year timeline for potential market availability, while lengthy, reflects the appropriate caution required for novel medical devices.
The broader implications extend beyond kidney stones to the field of magnetically guided therapeutic delivery systems. As medical robotics continues advancing alongside genetic engineering and AI-driven diagnostics, targeted intracorporeal interventions may become increasingly viable alternatives to systemic treatments or invasive procedures.
For patients suffering from recurrent uric acid kidney stones, this research offers hope for a future where dissolution replaces destruction, and precision replaces invasiveness, in medical treatment approaches.
Recommended Tools and Resources
To stay updated on the latest breakthrough technologies and scientific discoveries, we recommend these essential tools:
Video Creation & Content
- Fliki – Turn Text into Video (20% OFF) – Create engaging educational videos about scientific breakthroughs
- Pictory – AI Video Creation (20% OFF with Code: CuriosityAI) – Transform research articles into compelling visual content
Research & Analytics
- vidIQ – YouTube Growth & SEO – Track trending science topics and optimize content reach
- AdCreative.ai – AI-Powered Ad Designs – Design professional visuals for scientific content
Audio & Communication
- ElevenLabs – Realistic AI Voiceovers – Generate professional narration for educational content
- Beehiiv – Build Your Own Newsletter – Share scientific discoveries with your audience
Security & Research Tools
- Surfshark VPN – Online Privacy Tool – Secure access to international research databases
- NordVPN – Protected browsing for sensitive research
These tools help researchers, educators, and science communicators effectively share breakthrough discoveries with broader audiences while maintaining professional standards and security.